Figure 2. CD44 increases P-gp protein stability in yeast.
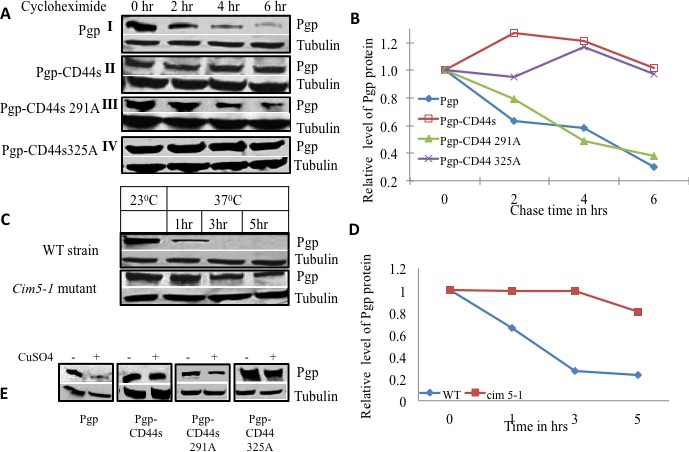
A. Yeast strains expressing either P-gp alone (I), P-gp and CD44 (II), P-gp and CD44 Ser291Ala mutant (III), or P-gp and CD44 Ser325Ala mutant (IV), were grown in overnight cultures, then treated with cycloheximide at a concentration of 10μg/ml for various times as indicated. Equal amounts of cell lysates from each treatment point were subjected to Western blot analysis using an anti-P-gp monoclonal antibody and an anti-alpha tubulin monoclonal antibody as loading control. B. The band intensities from blot A were quantified using densitometry. C. P-gp was expressed in the wild type yeast parent strain as well as in the conditional proteasome mutant cim5-1 at the permissive temperature (23°C) and later at the non-permissive temperature (37°C) where the proteasome is nonfunctional in the cim5-1 mutant. Aliquots were drawn at the respective time points as indicated and P-gp expression were evaluated by Western blot analysis using anti-P-gp along with alpha tubulin monoclonal antibodies D. The band intensities from blot C were quantified using densitometry. E. Yeast strains harboring P-gp with and without CD44 or its phosphorylation mutants were transformed with a myc-tagged ubiquitin plasmid with a CUP promoter. Cells were grown in the absence or presence of added CuSO4 as indicated. The expression of P-gp, and tubulin were studied by Western blot analysis using anti-P-gp, and anti-alpha tubulin monoclonal antibodies as labeled.
