Figure 3. CD44 protects P-gp from ubiquitination.
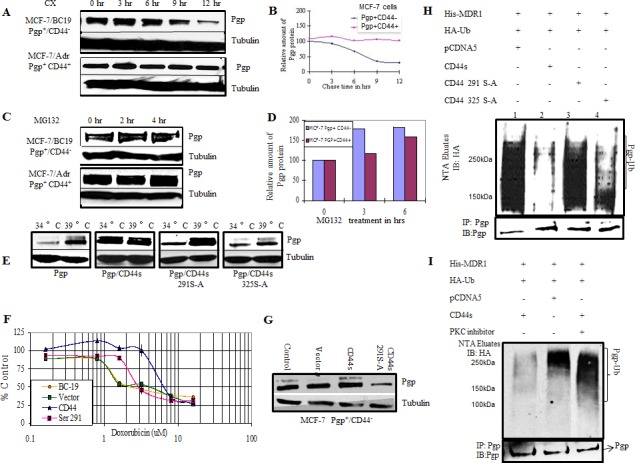
A. MCF-7/BC19 and MCF-7/Adr cells were treated with 50μg/ml cycloheximide. Cells were harvested at the designated time points. Total cell lysates were prepared and subjected to Western blotting analysis to detect the amount of P-gp present on the cells. Tubulin was used as a loading control. B. The band intensities from blot A were quantified using densitometry. C. MCF-7/BC19 and MCF-7/Adr cells were treated with the proteasome inhibitor MG 132 to a final concentration of 10μM for 2 hr and 4 hr as indicated. Total cell lysates were prepared and subjected to Western blot analysis probed with anti-P-gp and anti-tubulin monoclonal antibodies. D. The band intensities from blot C were quantified using densitometry. E. CD44 and phosphorylation mutants at residue 291 or 325 were stably expressed in ts20 3T3 cells that contained a temperature-sensitive defect in the E1 ubiquitin activating enzyme. P-gp was then transiently transfected in these cell lines and initially cultured at the permissive temperature (34°C) before being cultured at the restrictive (39°C) temperature (ubiquitination defective) for 18 hours. Cell lysates were collected and probed with anti-P-gp and anti-tubulin monoclonal antibodies. F. MCF-7/BC19 cells were transfected with either empty vector, full length CD44s or the CD44 Ser291Ala mutant, and treated with varying concentrations of doxorubicin. The MTT assay was performed and the viability of each cell line was plotted as percent of control against the concentration of drug used. G. Aliquots from each transfectant used on panel F at time zero were taken to perform Western blots and determine P-gp expression. H. In vivo ubiquitination experiments were performed as follows: HEK293T cells were co-transfected with His-tagged MDR1, HA-tagged ubiquitin and either wild-type CD44, CD44 Ser291Ala mutant, CD44 Ser325Ala mutant or empty vector as indicated. His-tagged P-gp was purified on Ni-NTA resin under denaturing conditions; the eluates were separated using SDS PAGE. The presence of ubiquitinated P-gp was determined by immunoblotting with anti-HA antibody. The lower panel demonstrates co-immunoprecipitation with anti-P-gp antibodies to determine the P-gp level of each transfectant. I. In vivo ubiquitination experiments were performed in the presence or absence of a PKC inhibitor as indicated. HEK293T cells were transfected with the indicated plasmids. After 48 hrs, the cells were treated with bisindolylmaleimide, a PKC inhibitor, for 4 hrs. The ubiquitinated proteins were purified on Ni-NTA resin under denaturing conditions and immunoblotted with anti-HA antibody. The upper panel shows the ubiquitinated species (P-gp-Ub). The lower panel represents co-immunoprecipitation with anti-P-gp antibodies to determine the P-gp level of each transfectant.
