Figure 2. Bim-induced apoptosis.
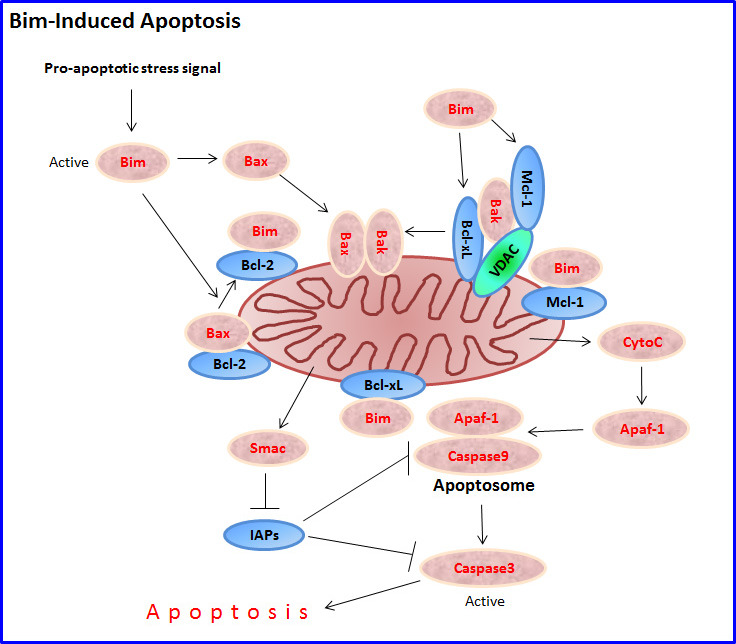
Following exposure to a pro-apoptotic stimulus, a sudden intracellular rise in free activated Bim molecules (e.g., by increased transcription and/or translation, increased alternative splicing in favor of BimS, and/or release of Bim from sequestered intracellular storages as a result of phosphorylation) initiates the intrinsic mitochondrial apoptotic pathway. Bim induces apoptosis by directly activating Bax and Bak, or indirectly by interacting with the anti-apoptotic proteins Bcl-2, Bcl-xL and Mcl-1, leading to the release and mitochondrial transfer of Bax and Bak. Under normal conditions, Bak is hold in check by Mcl-1, VDAC2 and Bcl-xL. Bax/Bak oligomerization in the mitochondrial outer membrane results in dissipation of the mitochondrial outer membrane potential (Δψm) and release of the apoptogenic proteins cytochrome C (CytoC), Smac/DIABLO and HtrA2 into the cytosol. Cytochrome C activates Apoptotic protease activating factor 1 (Apaf-1) that facilitates the formation of the apoptosome where caspase 9 is activated to initiate the apoptotic cascade concluded with the activation of caspase 3. Smac/DIABLO antagonizes the anti-apoptotic function of Inhibitors of apoptosis protein (IAPs) such as XIAP, cIAP1 and cIAP2, thereby enhancing apoptosis induction by cytochrome C.
