Abstract
Acclimating to toxicant stress is energy expensive. In laboratory toxicology tests dietary conditions are ideal, but not in natural environments where nutrient resources vary in quality and quantity. We compared the effects of additional lipid resources, docosahexaenoic acid (n-3; DHA) or linoleic acid (n-6; LA), or the effects of the toxicants, atrazine or triclosan on post-treatment starvation survival, reproduction, and lipid profiles. Chemical exposure prior to starvation had chemical-specific effects as DHA showed moderately beneficial effects on starvation survival and all of the other chemicals showed adverse effects on either survival or reproduction. Surprisingly, pre-exposure to triclosan inhibits adult maturation and in turn completely blocks reproduction during the starvation phase. The two HR96 activators tested, atrazine and LA adversely reduce post-reproduction survival 70% during starvation and in turn show poor fecundity. DHA and LA show distinctly different profiles as DHA primarily increases the percentage of large (>37 carbon) phosphatidylcholine (PC) species and LA primarily increases the percentage of smaller (<37 carbon) PC species. The toxicants atrazine and triclosan moderately perturb a large number of different phospholipids including several phosphatidylethanolamine species. Some of these polar lipid species may be biomarkers for diets rich in specific fatty acids or toxicant classes. Overall our data demonstrates that toxicants can perturb lipid utilization and storage in daphnids in a chemical specific manner, and different chemicals can produce distinct polar lipid profiles. In summary, biological effects caused by fatty acids and toxicants are associated with changes in the production and use of lipids.
Keywords: HR96, lipid profiles, metabolism, Daphnia, lipid allocation, development
1. Introduction
Acclimation to toxicants requires significant energy. The advent of global gene expression technologies has provided unique insights demonstrating numerous energy metabolism changes that occur following toxicant exposure. For example, Cr(VI) increases liver and heart-type FABP and glucose transporter, GLUT2, while repressing apolipoprotein B and cytochrome c oxidase expression in Fundulus heteroclitus liver (Roling et al., 2006). Carbon tetrachloride exposure leads to induction of genes related to phospholipid structure, lipid metabolism, and glycolysis in Oncorhynchus mykiss (Koskinen et al., 2004). Recent studies have shown that polyaromatic hydrocarbons and polychlorinated biphenyls disrupt mitochondrial function and oxidative phosphorylation in F. heteroclitus hepatocytes (Du et al., 2015), and the flame retardant Firemaster 550 (FM550), alters nutritional status in Daphnia magna by perturbing fatty acid, glucose, and amino acid metabolism (Scanlan et al., 2015).
Interestingly, toxicant responsive transcription factors such as AhR, CAR, and PXR that regulate phase I-III detoxification also regulate nutrient allocation and energy homeostasis (Ueda et al., 2002; Dong et al., 2009; Hernandez et al., 2009; Lu et al., 2015). For example, the AhR agonist TCDD alters the expression of genes involved in cholesterol biosynthesis, lipogenesis, and glucose metabolism (Sato et al., 2008). Phenobarbital activation of CAR down-regulates fatty acid oxidation and glucose synthesis, and reduces thyroid hormone activity by increasing thyroid hormone metabolism (Ueda et al., 2002; Maglich et al., 2004). PXR activation by PCN down-regulates β-oxidation and ketogenesis, and increases lipogenesis (Nakamura et al., 2007). CAR and PXR also cross talk with insulin or glucagon responsive transcriptions factors such as forkhead box O1 (regulates gluconeogenesis and glycogenolysis by insulin signaling), forkhead box A2 (regulates β-oxidation and ketogenesis), cAMP-response element binding protein (involved in gluconeogenesis), and peroxisome proliferator activated receptor gamma coactivator 1α (induces genes involved in mitochondrial oxidative metabolism) (Konno et al., 2008; Gao and Xie, 2012).
Daphnia and Drosophila HR96 receptors are orthologous to CAR/PXR/VDR receptors in mammals (Litoff et al., 2014), and aid in acclimation to toxicant stress (King-Jones et al., 2006; Sengupta et al., 2015). Drosophila HR96 has also been shown to regulate gastric lipase (Magro), Niemann Pick type C1, Acyl coenzyme A acyltransferase, and ABC transporter genes involved in cholesterol and triacylglycerol homeostasis and transport (Bujold et al., 2010; Sieber and Thummel, 2012). DHA represses both mammalian CAR and D. magna HR96 activity (Li et al., 2007; Lu et al., 2008; Karimullina et al., 2012), and linoleic acid (LA, n-6 fatty acid) activates both CAR and HR96 (Finn et al., 2009; Karimullina et al., 2012), indicating similarities between the mammalian and invertebrate receptors in their response to some lipids. In addition, triclosan, a commonly used antimicrobial agent in household products, represses HR96 activity, and atrazine, a triazine herbicide activates HR96 (Karimullina et al., 2012). Atrazine is known to cause adverse mixture responses with other pesticides (Pape-Lindstrom and Lydy, 1997; Chen et al., 2015), however, it may also provide protection from triclosan and unsaturated fatty acids in D. magna by inducing enzymes that provide protection from lipid peroxidation (Sengupta et al., 2015). Because oral exposure to toxicants and PUFAs may occur simultaneously, there may be competition for receptors. Therefore, toxicants may cause inappropriate lipid-like responses or alter HR96’s ability to respond to diet. Conversely, exposures to specific or changing diets may perturb an individual’s toxicant responses and chemical sensitivity (Karimullina et al., 2012; Ginjupalli et al., 2015; Leão et al., 2015).
PUFAs are crucial structural and regulatory lipids within phospholipid membranes. Alterations in dietary PUFA levels, n-3 fatty acids and n-3/n-6 ratio can also affect membrane-linked cellular processes involved in energy metabolism (Hulbert et al., 2005). Research indicates that changes in dietary lipids impact metabolic rate, neuropeptide activity, second messenger generation and gene expression thus affecting several glucose and lipid metabolism pathways; confirming the role of PUFAs as signaling molecules related to growth, development, and energy metabolism (Hulbert et al., 2005; Wei et al., 2010; Catala, 2013).
PUFA-rich phytoplankton are associated with better growth rate and fecundity in both invertebrates and vertebrates (Verreth et al., 1994; Brett and Muller-Navarra, 1997). PUFAs such as arachidonic acid (n-6 fatty acid), eicosapentaenoic acid (EPA, an n-3 fatty acid) and DHA (n-3) are considered important in the growth and reproduction of aquatic species, including arthropods (Brett and Muller-Navarra, 1997; Ginjupalli et al., 2015). D. magna growth may be directly attributed to increases in n-3 fatty acids such as α-linolenic acid, DHA and EPA; where α-linolenic acid is considered the primary n-3 producer and EPA is considered the primary final n-3 product (Becker and Boersma, 2005). D. magna is known to retain EPA and arachidonic acid during starvation or when food is scarce (Brett et al., 2006). Some studies indicate DHA is retained in the ovaries of daphnids (Bunescu et al., 2010), but most research indicates that DHA is rapidly converted to EPA in daphnids (Taipale et al., 2011).
Dietary restriction positively effects lifespan in some species (mice, C. elegans, D. melanogaster), however positive impacts of diet restriction are not prevalent in all species (Mair and Dillin, 2008; Latta IV et al., 2011). Under limited diet resources organisms tend to reduce reproduction because of its metabolic costs and invest in somatic maintenance for better survival until normal conditions are available for reproduction. This also explains the lifespan-extension observed in several organisms under dietary restriction (Mair and Dillin, 2008). D. magna is a suitable model for understanding life history trade-offs of dietary restriction as there is already a vast amount of literature available on relationship between food concentrations and daphnid life history strategies (Latta IV et al., 2011). There is also an increasing interest in understanding how diet plays a role in determining the toxicant sensitivity of different organisms (Pavlaki et al., 2014). For example, algal diet and lipid concentrations have been proposed to be confounding variables in pesticide-induced male production (Olmstead and LeBlanc, 2003; Ginjupalli et al., 2015). In a natural ecosystem, organisms are exposed to different kinds of diets at different times of the year. One such example is algal succession in freshwater ecosystems, which has an impact on the higher organisms of the food chain. This variation has the potential of acting as a biotic stressor as the diet may interact differently with key lipid receptors. It is important to understand interactions between stressors, and Daphnia with its well understood ecology, provides an excellent model for linking ecology or physiology to specific metabolic and genomic changes (Colbourne et al., 2011).
Our goal is to determine if environmental toxicants that perturb HR96 activity could alter resource allocation in D. magna. In part, we want to determine if D. magna would alter its resource allocation towards survival or reproduction during starvation stress depending on the chemicals to which they had been previously exposed, and test whether specific polar lipids are associated with specific chemicals or physiological responses. We tested whether lipids (DHA or LA) or toxicants (atrazine or triclosan) may alter resource allocation through starvation survival, starvation reproduction and polar lipid profiles.
2. Materials and Methods
2.1. Daphnia magna culture
D. magna were maintained at 21–23°C in moderately hard water at a pH of 8.1 – 8.3 in a 16:8 light:dark cycle and fed cultured Pseudokirchneriella subcapitata (Aquatic Biosystems, Fort Collins, CO USA) supplemented with TetraFin fish food (Masterpet Corp., New South Wales, Australia) as described previously (Ginjupalli and Baldwin, 2013).
2.2. Chemicals
LA (≥ 99 %), DHA (≥ 98 %) and triclosan (97%) (Sigma-Aldrich, St. Louis, MO USA) stock solutions were dissolved in absolute ethanol (Sigma-Aldrich Chemical Co., Inc, Milwaukee, WI USA). Atrazine (98.9%) (Sigma-Aldrich, St. Louis, MO USA) stock solution was dissolved in 99.7% DMSO (Fisher Scientific, Fair Lawn, NJ, USA).
2.3. Chronic toxicity tests
The reproductive toxicity of DHA, LA, atrazine and triclosan were determined by exposing <24-h neonates (n=10) to these chemicals at multiple concentrations in 40 ml of culture medium that was renewed every other day for 21 d ((ASTM), 1988; Baldwin et al., 2001). Daphnids were fed 3 × 106 P. subcapitata cells supplemented with 50 μl of an aqueous suspension of blended, TetraFin fish flakes at 2.5 mg/ml dry weight 2X per day as described previously (Baldwin et al., 1997; Ginjupalli et al., 2015). Chemical concentrations used in the chronic toxicity tests were determined based on previously published acute toxicity tests performed in our laboratory (Sengupta et al., 2015). Atrazine was reconstituted in 99.7% DMSO for a total of 0.016% DMSO in each exposure beaker including the solvent-only (untreated; UT) group. DHA, LA and triclosan were reconstituted in 100% ethanol for up to a total of 0.004% ethanol in each exposure beaker including the UT group. Differences in reproduction were determined by one-way ANOVA followed by Fisher’s Least Significant Difference as the post-hoc test with a p-value of 0.05 considered significant. Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA USA).
2.4. Starvation assay
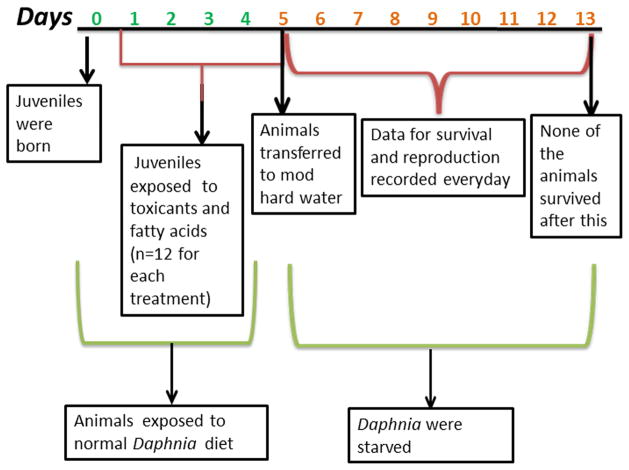
D. magna (<24 h old) were fed and exposed to chemical treatments, LA (4 and 8 μM), DHA (2 and 4 μM), atrazine (20 and 40 μM) and triclosan (0.25 μM) (n=12) as described above. On day 5, chemical exposure and feeding was stopped (Fig 1). Starvation survival and reproduction in the adolescent daphnids was monitored up to day 13 as none of the animals survived past day 13. Differences in reproduction were determined by one-way ANOVA with a general linear model followed by Fisher’s Least Significant Difference test with a p-value of 0.05. Differences in survival were determined by Fisher’s exact test.
Fig. 1.
Outline of the experimental design and timeline for the treatments and starvation of daphnids as described in the Materials and Methods.
2.5. Cholesterol analysis
Treated and untreated daphnids were collected and homogenized in 100 μl of a pH 7.4 0.01M HEPES, 0.05M EDTA, 10% glycerol buffer. Tubes were centrifuged at 10,000 rpm for 1 minute, and supernatant cholesterol concentrations were determined using a commercially available fluorometric assay kit (Cayman Chemical Co., Ann Arbor, MI USA) at an excitation wavelength of 550 nm and an emission wavelength of 590 nm (Amundson and Zhou, 1999).
2.6. Lipid extraction and Lipidomic analysis
Daphnids were collected at 5-days old (prior to starvation; see Fig 1) for lipid extraction and lipidomic analysis. Polar lipids were extracted from 5 tubes of 5 juvenile daphnids using previously published protocols (Fitzgerald et al., 2007) adapted to D. magna. Daphnids were homogenized and shaken in a 1 ml, 1:1 mix of water:chloroform. Following layer separation, 1.5ml of a 1:2 solution of chloroform:methanol was added and vortexed. The solution was centrifuged for 1 minute at 600 rpm, and the lower layer was collected using a glass Pasteur pipette. Chloroform addition (0.5 ml) followed by vortexing, centrifugation, and collection was performed two more times. The lipid extract was cleaned with subsequent washes of 1M KCl (0.5ml) and water. The lower layers were collected, placed in a teflon covered glass vial and dried under nitrogen. Samples were then frozen with dry ice and sent to the Kansas Lipidomics Research Center for lipidomic analysis. Lipid profiles were determined by mass spectrometry (electrospray ionization triple quadrupole mass spectrometer from Applied Biosystems API 4000) at Kansas Lipidomics Research Center as described previously (Isaac et al., 2007).
Changes in overall lipid profiles were determined by two-way ANOVA with repeated measures and the Bonferonni post-hoc test. One-way ANOVA followed by Tukey’s post-hoc test was used to investigate differences between treatment groups for each of the individual polar lipid species. In addition, hierarchical clustering combined with one-way ANOVA with a p-value cutoff of 0.01 was used to cluster and visualize significant changes in the concentrations of individual lipids with MultiExperiment Viewer. PCA was performed with SAS 9.3 to show and confirm associations between specific lipids and chemical treatments (SAS Institute Inc., Cary, NC USA).
3. Results
3.1. Reproductive toxicity during 21-day chemical exposures
Reproductive fitness was determined in D. magna exposed to atrazine, triclosan, DHA, and LA. DHA, LA and triclosan did not reduce reproduction at any of the concentrations tested. However, atrazine reduced reproduction by 50% – 90% at concentrations from 10 – 80 μM (Suppl File 1) with little effect (14%) at 5 μM. Previous studies found that atrazine reduced reproduction almost 30% at 2.32 μM (0.5 mg/L) and greater than 80% at 69.55 μM (15 mg/L) (Palma et al., 2009). Therefore, our data for atrazine is similar but not identical to what has been previously reported. We observed a concentration-dependent drop in reproduction during triclosan exposures from 0.022 – 0.345 μM, but it was not statistically significant by ANOVA. Previous studies reported 0.22 μM (64 μg/L) triclosan reduced reproduction during chronic toxicity tests with D. magna (Peng et al., 2013). To our knowledge, this is the first paper to report the reproductive toxicity of DHA or LA (Suppl File 1).
Subsequent research with each chemical was performed at concentrations that did not significantly alter reproduction based on our chronic toxicity tests for DHA (2–4 μM), LA (4–8 μM), and triclosan (0.25 μM). We continued to use 20 – 40 μM atrazine despite the fact these concentrations reduced fecundity because these concentration have been shown to protect D. magna from the toxic effects of other chemicals such as triclosan and DHA most likely because of HR96 activation (Sengupta et al., 2015).
3.2. Chemical-induced changes in starvation survival and fecundity in D. magna
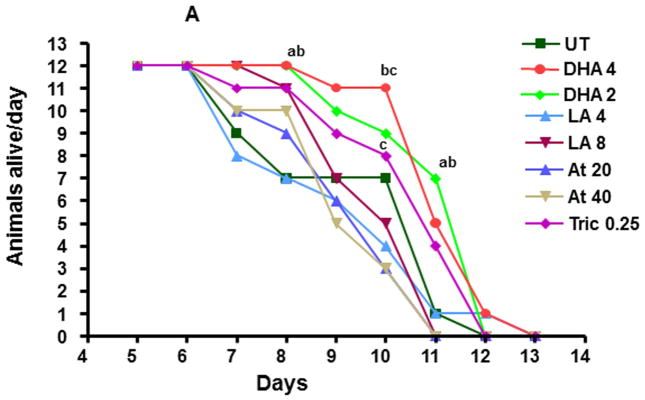
We hypothesized that several endogenous and xenobiotic chemicals, especially those that increase HR96 activity, may perturb resource allocation leading to increased survival during periods of starvation because of increased lipid absorption (King-Jones et al., 2006; Horner et al., 2009). Therefore, daphnids aged 1–5 days old were exposed to either HR96 activators (atrazine, LA) or HR96 inhibitors (triclosan, DHA) (Karimullina et al., 2012) and then starved to determine chemical-induced changes in starvation survival and reproduction. Survival rarely varied between UT daphnids and the other groups with the exception of day 8 and day 11 in which DHA-treated daphnids showed greater survival than untreated daphnids. However, daphnids exposed to DHA did show increased survival relative to daphnids exposed to LA and atrazine (Fig. 2). In general there was a pattern indicating better survival for DHA > triclosan > LA > atrazine. This is the opposite of what we hypothesized as both HR96 inhibitors (triclosan and DHA) showed better starvation survival compared to the HR96 activators (atrazine and LA).
Fig. 2. Starvation survival in D. magna following exposure to DHA, LA, atrazine, or triclosan.
Data are presented as the number of daphnids alive each day. Survival of adolescent daphnids was analyzed using Fishers 2×2 test (n=12). An ‘a’ represents different from UT, ‘b’ different from LA (4 μM) and ‘c’ different from Atr (20 and 40 μM) groups.
Reproduction was split into three tiers: groups that reproduced well, those that reproduced moderately well, and those that reproduced poorly during starvation (Table 1). The DHA groups and UT group reproduced the best (Table 1). Therefore, DHA showed some benefit in both starvation survival and reproduction relative to the other treatment groups (Fig. 2; Table 1). We expected that atrazine would demonstrate poor reproduction based on the chronic toxicity test data (Suppl File 1) and it did; however, reproduction was even worse in triclosan- and 4μM LA-exposed daphnids. Interestingly, the triclosan-exposed daphnids did not reproduce during starvation and appeared to enter a senescence or quiescence in which they no longer continued to develop. Other treatment groups continued to develop into adults despite the lack of food.
Table 1.
Survival of daphnids following release of broods.
| Treatment | Neonates/Daphnid# | Adults that reproduced | Adults that survived after reproduction |
|---|---|---|---|
| UT | 8.83 ± 3.12a | 7 | 7 |
| 2 μM DHA | 11.42 ± 1.92a | 12* | 9 |
| 4 μM DHA | 8.92 + 1.29a | 12* | 11 |
| 4 μM LA | 2.17 ± 1.16c | 3 | 1 |
| 8 μM LA | 7.83 + 1.91ab | 9 | 3* |
| 20 μM Atr | 3.83 + 0.99b | 8 | 2* |
| 40 μM Atr | 4.00 + 1.13b | 7 | 2* |
| 0.25 Tric | 0.00 + 0.00c | 0* | 0 |
n = 12 per treatment group
Letters that are different indicate statistical significance from other groups via ANOVA with a general linearized model followed by Fisher’s LSD.
indicates statistically different from UT group as determined by Fisher’s 2×2 test.
During starvation, several of the adult female daphnids died within 24 hours of releasing a brood (Table 1). A significant drop in post-reproductive survival (approaching 70%) was observed in the atrazine and LA treated groups; both HR96 activators. This suggests an allocation of resources towards reproduction and away from survival in the daphnids treated with these HR96 activators. This is in contrast to triclosan-treated daphnids that did not reproduce and instead remained in the adolescent stage during the entire starvation phase (up to Day 13), and the DHA-exposed daphnids that survived following the release of their first brood.
3.3. Chemical exposures alter cholesterol levels and polar phospholipids
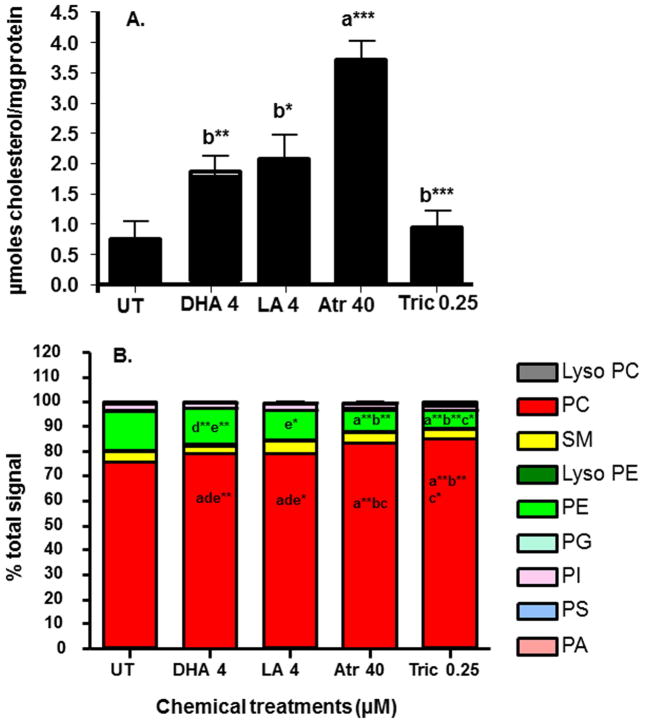
HR96 is a potent magro inducer in Drosophila (Sieber and Thummel, 2009). Magro, also known as gastric lipase in mammals, hydrolyzes cholesterol esters and promotes cholesterol clearance from the intestine (Sieber and Thummel, 2012). Therefore, we hypothesized that HR96 activators would reduce cholesterol. However, atrazine-exposure increased cholesterol concentrations greater than all other groups and the other HR96 activator, LA, produced the 2nd highest cholesterol concentrations. All chemical exposures increased cholesterol concentrations in the homogenized daphnids relative to control daphnids (Fig. 3A).
Fig. 3. Cholesterol concentrations and relative polar lipid levels in daphnids following four days of exposure to different chemicals.
(A) Cholesterol concentrations in homogenized daphnids. Data are presented as mean ± SEM. Data was analysed by one-way ANOVA followed by Tukey’s multiple comparison test (n=6) (*p<0.05, **p<0.01 and ***p<0.001). An ‘a’ is different from UT and ‘b’ is different from Atr. (B) Relative polar lipid levels from chemically-exposed daphnids. Data were analyzed by two-way ANOVA (with repeated measures) followed by Bonferroni post-hoc test (n=5). ‘a’ is different from UT, ‘b’ is different from DHA, ‘c’ is different from LA, ‘d’ is different from Atr and ‘e’ is different from Tric (p<0.05, *p<0.01, **p<0.001).
In part because cholesterol did not drop as expected and in part because we suspected that HR96 regulators and lipids alter lipid profiles, we examined changes in several polar lipid classes. Total lipids were significantly lower in toxicant-exposed groups (atrazine and triclosan); reflected primarily by a small drop in PC and larger drop in PE (Table 2). To take a closer look at relative levels of individual lipid species, samples were evaluated as percent total signal. PC comprised 75% and PE 15.5% of the lipidome as these two polar lipids make up greater than 90% of the polar lipids in D. magna. SM (4.4%), PI (2.8%), LysoPE (0.74%), PS (0.57%), LysoPC (0.52%), PG (0.44%), and phosphatidic acid (0.08%) comprise the remaining polar lipid species (Fig. 3B). Again, PC and PE were significantly altered following chemical exposures. Percent total PC was increased in all groups compared to the untreated daphnids, and the percent PC in the toxicant exposures (atrazine, triclosan) was higher than in the PUFA exposures (LA, DHA) (Fig. 3B). Consequently, percent total PE was reduced about 2X in the atrazine and triclosan groups (Fig. 3B).
Table 2.
Quantity of each lipid type found in the polar lipids extracted from treated and untreated D. magna.
| Lipid | UT# | DHA (4μM) | LA (4μM) | Atr (40 μM) | Tric (0.25μM) |
|---|---|---|---|---|---|
| LysoPC | 0.230±0.068 | 0.149±0.111 | 0.125±0.026 | 0.074±0.032 | 0.081±0.046 |
| PC | 33.694±7.536 | 28.955±7.453a | 25.524±4.164a | 18.552±3.291a | 12.805±7.230a |
| SM | 1.951±0.431 | 1.114±0.248 | 1.368±0.319 | 0.985±0.180 | 0.669±0.531 |
| LysoPE | 0.327±0.103 | 0.224±0.053 | 0.145±0.043 | 0.069±0.043 | 0.055±0.056 |
| PE | 7.045±2.102 | 5.252±1.532 | 3.517±0.871 | 2.131±1.507b | 1.463±1.771b |
| PG | 0.205±0.100 | 0.104±0.051 | 0.081±0.028 | 0.057±0.018 | 0.036±0.018 |
| PI | 1.258±0.337 | 0.701±0.231 | 0.723±0.174 | 0.422±0.067 | 0.349±0.304 |
| PS | 0.257±0.058 | 0.179±0.044 | 0.203±0.039 | 0.196±0.052 | 0.152±0.049 |
| PA | 0.038±0.016 | 0.023±0.006 | 0.024±0.012 | 0.015±0.004 | 0.014±0.009 |
| Lipids | 45.005±10.395 | 36.700±9.530 | 29.709±5.206 | 22.501±4.600b | 15.624±9.755bc |
Data presented as mean nmol lipid/sample +/− standard error.
Statistical differences determined by two-way ANOVA followed by the Bonferroni post-hoc test (n=5).
refers to different from all other treatments,
is different from UT,
is different from DHA.
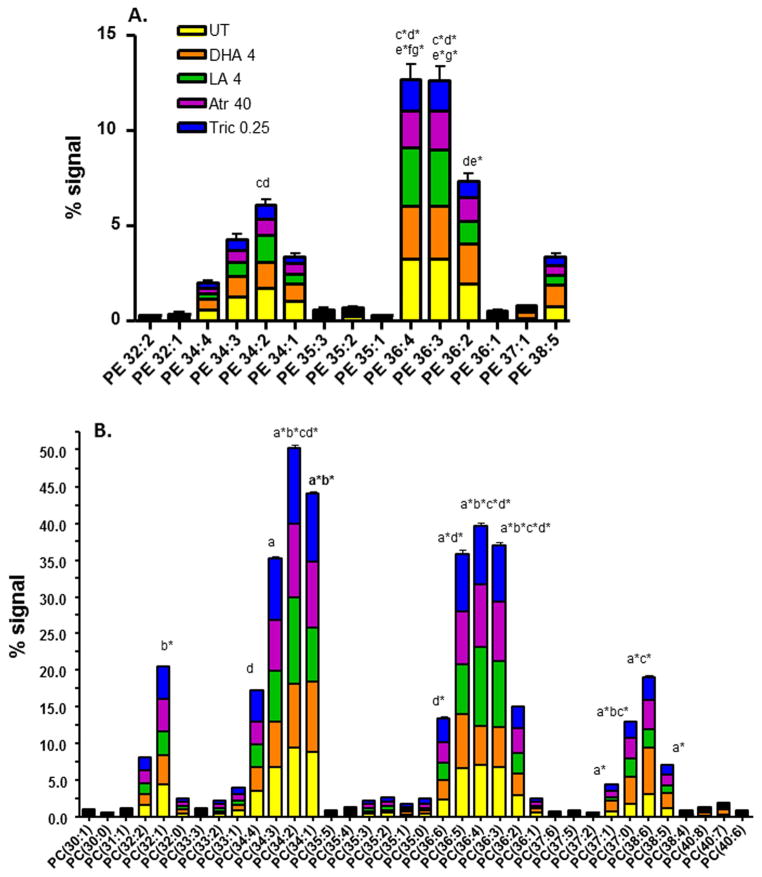
Therefore, we investigated the relative percent total signal for the individual PE and PC species (Fig. 4). Compared to the untreated daphnids there are few changes in the PE species with the exception of the 34 and 36 carbon PE species that are significantly lower in the atrazine and triclosan groups (Fig. 4A, Suppl File 3). However, there are many changes in individual PC species between UT and chemical exposures (Fig. 4B) and between the different chemical exposure groups (Suppl File 4). The percent signal for PC 34:2, 36:3, and 36:4 are perturbed in all groups compared to the control, and PC 37:0 is different from the controls in all groups except triclosan. Several large PC species (40:7, 38:5, 37:1) are only different from the controls following DHA exposure. Triclosan specifically perturbed PC 34:4 and 36:6 (Fig. 4B; Suppl Files 4–5).
Fig. 4. Changes in phosphatidylethanolamine (PE) (A) or phosphatidylcholine (B) composition among different exposure groups.
Changes in percent signal of individual PE (A) or PC (B) species following chemical exposures (UT = control, DHA 4 = 4 μM DHA, LA 4 = 4 μM LA, Atr 40 = 40 μM atrazine, Tric 0.25 = 0.25 μM triclosan) in daphnids were analyzed by two-way ANOVA (with repeated measures) followed by the Bonferroni post-hoc test. Statistical differences between different groups are noted with letters; ‘a’ indicates UT vs DHA, ‘b’ is UT vs LA, ‘c’ is UT vs Atr, ‘d’ is UT vs Tric, ‘e’ is DHA vs Tric, ‘f’ is LA vs Atr and ‘g’ is LA vs Tric (only letter = p<0.01, * indicates p<0.001).
SM, PI, PE and PS species also showed individual differences relative to controls or other groups (Suppl File 2). SM 38:1 is decreased in the atrazine, triclosan, and DHA groups, PI 36:2/3/4 are increased by several treatments including DHA, PI 34:2 is increased only by DHA, and PS 36:2, and 36:4 are increased by triclosan.
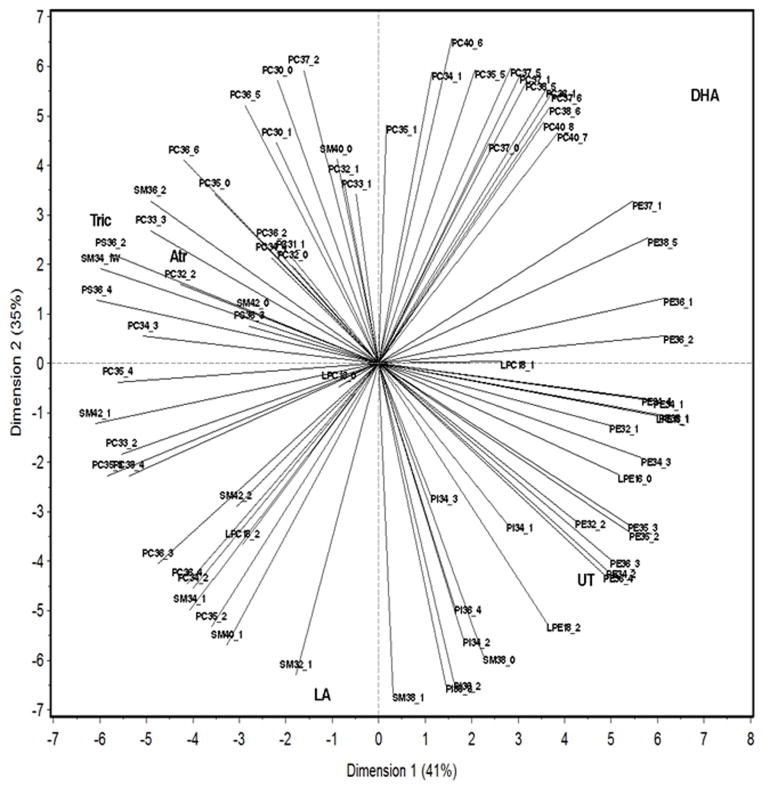
PCA was performed because two-way ANOVAs (Fig. 4; Suppl Files 2, 4) indicated associations between specific polar lipids and chemical exposures. Specific lipid species are associated with the different treatments. PCA demonstrates that the atrazine and triclosan groups are most similar while the DHA and LA groups often show opposing lipid profiles (Fig. 5). In addition, PCA confirmed that several large PC species such as 36:1, 37:1, 37:5, 37:6, 38:5, 38:6, 40:7, and 40:8 are associated with DHA exposure. Some SM, PI, and mid-weight PC species such as SM 32:1, 38:1, 40:1, 34:1, PI 36:2, 36:4, 34:2, and PC 36:4, 36:3, 34:2, 35:2 are associated with LA exposure. PS 36:2, 36:4, SM 42:0, 34:1, 36:2, and PC 34:3, 33:3, 32:2, 35:0, 36:6 are associated with triclosan and atrazine exposure.
Fig. 5. Principle component analysis reveals associations between chemical exposures and lipid profiles in D. magna.
Variability among polar lipids was observed following chemical treatment.
PCA and two-way ANOVA indicate distinct lipid groupings based on the different chemical exposures (Figs. 4–5). Therefore we performed hierarchical clustering of the different treatments and polar lipids coupled with one-way ANOVA to filter non-significant polar lipids and better show specific patterns of polar lipids (Suppl File 6). The hierarchical clustering confirms the similarities of atrazine and triclosan exposures, and demonstrates a large difference between DHA exposure and the other exposures as DHA clusters into a separate clade from the other exposure groups. Scatter plots also confirm significant changes in specific polar lipid species (Suppl Files 3,5,7). For example, DHA caused the most changes in PC species with significant increases in large PC species such as 36:1, 37:0, 37:1, 37:5, 37:6, 38:5, 38:6, 40:7, and 40:8. PC 37:0, 37:1, 38:5, 38:6, 40:7, and 40:8 are either major PC species or show relatively large changes (>2X) following DHA exposure. ANOVA also confirmed the PCA data indicating that LA and DHA cause opposing actions on the relative abundance of several phospholipids (Suppl File 5, Fig. 5). The change in concentration of some of these PC species may provide a novel biomarker for DHA or LA exposure or function.
Atrazine and triclosan showed similar lipid profiles. Each altered the relative concentrations of 15 different phospholipids with 8 of the changes being identical in the atrazine/triclosan groups (Suppl Files 3,5,7). Most of the polar lipids that show differences between control and triclosan or control and atrazine were within the PCs (Suppl File 5). Overall there were more significant changes in polar lipids caused by the toxicant exposure groups (atrazine and triclosan) in comparison to the control group than the PUFA groups (LA and DHA) compared to the control group; however, changes were usually larger in the PUFA exposed groups.
DISCUSSION
Each of the chemicals tested induced unique lipidomic signatures in the daphnids (Fig. 5, Suppl File 6). For example, LA and DHA had its most prominent effects on relative abundance of PC species with LA predominantly effecting PCs 36 carbons or less and DHA predominantly effecting PCs 37 carbons or more (Figs. 4–5; Suppl File 5). In addition, LA and DHA had nearly diametrically opposed placement following the PCA (Fig. 5). The two toxicants tested, triclosan and atrazine showed similar signatures to each other (Fig. 5) primarily because each significantly perturbs eight polar lipids in nearly identical fashion including significant decreases in several PE and PI species (Fig. 5, Suppl Files 3,7). Still triclosan and atrazine showed unique features from each other as atrazine preferentially increased the relative abundance of 36–38 carbon PC species, while triclosan reduced mid-range PS (36:2, 36:4) and PC (34:4, 36:4) species (Suppl Files 3,5,7).
It is possible that one or more of these polar lipid species may become biomarkers of exposure to a specific type of chemical, or a biomarker associated with a specific effect. Of special interest is triclosan. Triclosan caused an interesting phenotype during starvation. In the standard 21-day chronic toxicity test, triclosan had no significant effect on reproduction (Suppl File 1). However, triclosan exposure prior to starvation caused starved daphnids to enter a senescent state as the triclosan-exposed daphnids did not develop beyond adolescence and therefore never reproduced. This effect was specific to triclosan only in our study, where all the other groups developed into reproductive adults even in the absence of food. The lack of reproduction following triclosan exposure could have devastating effects on a stressed pond population.
Recent research indicates that triclosan inhibits fatty acid synthase (FASN) (Sadowski et al., 2014) and this may contribute to the repressed development as lipid stores may not have been produced or available for growth and development. Polar lipid concentrations were lower in triclosan exposed daphnids (Table 2). However, survival was not lower than the other groups indicating lipids were available for survival, but not reproduction and development. Several polar lipids were repressed by triclosan exposure including multiple PE species (Fig. 4–5; Suppl File 3). Triclosan (and atrazine) moderately decreased PE 36:2, which has previously been associated with diabetes (Lappas et al., 2015). To a lesser extent some PI, PS, and PC species were also perturbed (Suppl Files 3, 5, 7) by triclosan exposure. However none of these lipid species are specific to triclosan as other chemicals that did not cause senescence also perturbed their relative abundance.
Other examples of unique signatures include DHA and LA. DHA’s effects on polar lipids were consistent across all analyses (Figs. 4–5; Suppl Files 2–7) with several large PC species highly increased by DHA including PC 37:0, 37:1, 38:6, 40:8 and to a lesser extent 37:5, 37:6, 38:5 (Figs. 4–5; Suppl Files 4, 5). The effects of LA on lipid profiles are not as unidirectional for the PC species. Smaller species with one double bond (PC 30:1, 32:1, 34:1) are decreased by LA exposure, and mid-sized PC species with multiple double bonds are primarily increased (PC 34:2, 35:2, 36:3, 36:4) by LA exposure (Figs. 4–5, Suppl File 5). LA has been used to protect embryos during cryogenic freezing and the high production of 36 and 38 carbon PC is hypothesized to be involved in this protective effect (Leão et al., 2015). Interestingly, in humans an increase in PC 36:4, a major supplier of arachidonic acid to the blood (Uhl et al., 2015), is associated with type I diabetes (Wittenbecher et al., 2015). PC 36:4 was increased by LA and atrazine, but decreased by DHA. DHA appears to be protective from diabetes and other metabolic diseases (Wei et al., 2010; Bhaswant et al., 2015), and atrazine is associated with mitochondrial dysfunction and obesity (Mokdad et al., 2001; Lim et al., 2009).
DHA is the only chemical that improved physiological endpoints measured during starvation relative to controls. Therefore, the reduction of PC 36:4 and the increase in large molecular weight PCs and PE 37:1 by DHA are associated with better starvation survival and reproduction. However, more work needs to be done to better define these associations. A much greater percentage of DHA-exposed daphnids successfully reproduced compared to the other groups (Table 1). In most exposure groups, reproduction during the starvation phase of the study often led to death of the reproducing adult, however the DHA-exposed adults showed better survival following their first brood than the other groups (Table 1). The higher number of adult daphnids that reproduced and the ability of some DHA-exposed daphnids to produce a second brood are key factors in the increased reproduction in DHA-exposed daphnids.
In contrast, triclosan, atrazine, and LA adversely affected starvation survival or reproduction (Fig. 2). Only atrazine reduced fecundity during the 21-day chronic toxicity test compared to untreated daphnids (Suppl File 1), therefore the other chemicals clearly show greater toxicity to daphnids under the stressful starvation conditions (Fig. 2, Table 1). Post-reproduction survival was significantly reduced (up to 70 %) by atrazine and LA (both HR96 activators) (Karimullina et al., 2012) exposure during starvation (Table 1). Chemical toxicity tests are performed under ideal conditions where temperatures are stable, light-cycles are optimal, and food is plentiful. However, food content may change because of temperature and seasonal changes leading to algal succession (Schlechtriem et al., 2006; Ginjupalli et al., 2015). These changes may alter the lipid and protein content available (Bergman Filho et al., 2011) and in turn perturb the nutritional content in the algae, altering energy budget, lipid profiles, and potentially increasing chemical toxicity (Pieters et al., 2006). Therefore, chemical toxicity may be greater than expected in natural environments because of seasonal changes that perturb algal succession and nutritional content. Of special concern is triclosan.
The percentage of PC was higher in all exposure groups relative to controls with the triclosan and atrazine groups showing the highest percent PC, 83 and 84.5% respectively. This occurred at the expense of PE (Fig. 3), and is in part why several PE’s are associated with the UT group (Fig. 5). DHA and LA have been shown previously to preferentially incorporate into phosphatidylcholines (Bouroudian et al., 1990; Leão et al., 2015). This may help explain the increase in PC following lipid exposures; however, this does not explain the increase in PC in the atrazine and triclosan groups.
The increase in PC may be a protective mechanism as phosphatidylcholines are associated with protection of various organs. An increase in PC is associated with increased survival of cryopreserved embryos (Leão et al., 2015), and reduced liver toxicity following exposure to alcohol, carbon tetrachloride, high-fat diet, and other toxicants primarily associated with oxidative stress (Aleynik et al., 1997; Lieber et al., 1997). However, CCl4-induced steatosis in rats causes an overall decrease in both hepatic PE and PC with an increase in specific species such a PE 34:1 that is decreased following LA, atrazine, and triclosan exposure in our study (Fig. 4; Suppl File 3), and PC 38:5 and 40:7 that are increased in severe steatosis (Ahn et al., 2008) and by atrazine or DHA exposure in our study (Fig. 4; Suppl File 5).
A recent study with JEG-3 cells demonstrated several changes in lipid profiles following exposure to tributyltin and perfluorinated chemicals including an increase in several PC species; however, few of these changes are similar to what we observed (Gorrochategui et al., 2014). A recent paper with D. magna also indicates that tributyltin disrupts PC incorporation into eggs (Jordão et al., 2015). There have been few published studies investigating the effects of toxicants on lipid profiles, and only two previous studies with D. magna (Jordão et al., 2015; Scanlan et al., 2015). Therefore, it is difficult to draw conclusions, but overall the data indicates there are clearly distinct lipid profiles between species, tissues, disease states, or mediated by chemical exposures and it appears that the incorporation of more PC into polar lipids may be a protective mechanism.
The two HR96 activators, LA and atrazine showed a similar physiological response with starved animals dying shortly after releasing their first brood. This suggests that their energy resources were put into reproduction and not survival. However, there are few obvious patterns between the two chemicals that associate specific lipids with a decrease in post-reproduction survival with the possible exception of some mid-weight PC (36:3, 36:4, 37:0) and PE (37:1, 38:5) species (Suppl File 3, 5, 6). Similarly, there are no lipids that can be easily associated with triclosan’s inhibitory effects on reproductive maturity. Overall, there are lipid profiles specific to the chemical exposures; however there are few lipids that can be easily associated with a specific phenotype at this time.
Furthermore, the two HR96 activators do not have similar lipid profiles (Fig. 5). Similarly, the two HR96 inverse agonists, DHA and triclosan only have a few lipids in common (Fig. 5, Suppl Files 3, 5, 7) such as PE 34:1, 38:5, and PC 36:3, 36:4, suggesting modes of action on lipid profiles are not related to HR96. Interestingly, PI 34:2, 36:2, 36:3, 36:4 are all modulated in a similar pattern by DHA, atrazine, and triclosan (Suppl File 6–7). PI’s are very important in signal transduction (Kim et al., 2015), but the specific processes altered if any are not known. While we expect incorporation of lipids to vary greatly between toxicants and lipids as DHA and LA can directly incorporate into lipids, this data suggests that HR96 is not mediating many of the lipid profile perturbations observed. In further support of this conclusion, the HR96 inverse agonist, triclosan, and the HR96 activator, atrazine have similar profiles as observed from the PCA (Fig. 5) and hierarchical clustering (Suppl File 6).
In summary, daphnids exposed to different dietary unsaturated fatty acids or environmental chemicals show diverse and chemical-specific lipid profiles. These profiles or specific lipids may be good biomarkers of exposure or be associated with disease or adverse effects, but these associations are weak or unknown at this time. Lipid profiles are not well associated with HR96 activity. Profiles do show starkly different profiles between the n-6 fatty acid LA and the n-3 fatty acid DHA and similar profiles between the two toxicants, atrazine and triclosan. Chemical exposure also caused unique responses under starvation conditions with DHA providing some survival benefits, atrazine and LA exposed daphnids that reproduce at the expense of the mother’s survival, and triclosan enhancing survival at the expense of reproduction. This demonstrates that toxicants can alter energy or lipid utilization and storage in daphnids by chemical specific means. Further experiments will attempt to discern developmental differences between neonates and adults that may provide clues as to why triclosan inhibits adult maturation. In conclusion, chemical exposure causes unique lipid profiles.
Supplementary Material
Highlights.
Chemical exposures alter D. magna’s starvation stress responses
Triclosan inhibits adult maturation and blocks reproduction during starvation
DHA supplementation increases starvation survival and does not perturb reproduction
The ratio of phosphatidylcholines to phosphatidylethanolamines was perturbed in all groups
Each chemical exposure induced specific changes in the profiles of polar lipids
Acknowledgments
Funds for this project were provided by NIH grant ES017321 to WSB.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ANOVA
analysis of variance
- CAR
constitutive androstane receptor
- DHA
docosahexaenoic acid
- DMSO
dimethyl sulfoxide
- FABP
fatty acid binding proteins
- FASN
fatty acid synthase
- FM550
Firemaster 550
- GLM
general linear model
- GLUT
glucose transporter
- LA
linoleic acid
- LSD
least significant difference
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PCA
principle component analysis
- PCN
pregnenolone 16 alpha-carbonitrile
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
- PUFA
polyunsaturated fatty acids
- PXR
pregnane-X-receptor
- SM
sphingomyelin
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- UT
untreated
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ASTM. Annual Book of ASTM Standards. ASTM; Philadelphia, PA: 1988. Standard guide for conducting renewal life-cycle toxicity tests with Daphnia magna; pp. 1–17. [Google Scholar]
- Ahn EJ, Kim H, Chung BC, Kong G, Moon MH. Quantitative profiling of phosphatidylcholine and phosphatidylethanolamine in a steatosis/fibrosis model of rat liver by nanoflow liquid chromatography/tandem mass spectrometry. J Chromatogr A. 2008;1194:96–102. doi: 10.1016/j.chroma.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Aleynik SI, Leo MA, Ma X, Aleynik MK, Lieber CS. Polyenylphosphatidylcholine prevents carbon tetrachloride-induced lipid peroxidation while it attenuates liver fibrosis. J Hepatol. 1997;27:554–561. doi: 10.1016/s0168-8278(97)80361-3. [DOI] [PubMed] [Google Scholar]
- Amundson DM, Zhou MJ. Fluorometric method for the enzymatic determination of cholesterol. Biochem Biophys Meth. 1999;38:43–52. doi: 10.1016/s0165-022x(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Baldwin WS, Bailey R, Long KE, Klaine S. Incomplete ecdysis is an indicator of ecdysteroid exposure in Daphnia magna. Environ Toxicol Chem. 2001;20:1564–1569. [PubMed] [Google Scholar]
- Baldwin WS, Graham SE, Shea D, LeBlanc GA. Metabolic androgenization of female Daphnia magna by the xenoestrogen 4-nonylphenol. Environ Toxicol Chem. 1997;16:1905–1911. [Google Scholar]
- Becker C, Boersma M. Differential effects of phosphorus and fatty acids on Daphnia magna growth and reproduction. Limnol Oceanogr. 2005;50:388–397. [Google Scholar]
- Bergman Filho TU, Soares AM, Loureiro S. Energy budget in Daphnia magna exposed to natural stressors. Environ Sci Pollut Res Int. 2011;18:655–662. doi: 10.1007/s11356-010-0413-0. [DOI] [PubMed] [Google Scholar]
- Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J Nutr Biochem. 2015;26:571–584. doi: 10.1016/j.jnutbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Bouroudian M, Nalbone G, Grynberg A, Leonardi J, Lafont H. In vitro study of docosahexaenoic acid incorporation into phosphatidylcholine by enzymes of rat heart. Mol Cell Biochem. 1990;93:119–128. doi: 10.1007/BF00226183. [DOI] [PubMed] [Google Scholar]
- Brett M, Muller-Navarra D. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biol. 1997;38:483–499. [Google Scholar]
- Brett MT, Muller-Navarra DC, Ballantyne AP, Ravet ML, Goldman CR. Daphnia fatty acid composition reflects that of their diet. Limnol Oceanogr. 2006;51:2428–2437. [Google Scholar]
- Bujold M, Gopalakrishnan A, Nally E, King-Jones K. Nuclear receptor DHR96 acts as a sentinel for low cholesterol concentrations in Drosophila melanogaster. Mol Cell Biol. 2010;30:793–805. doi: 10.1128/MCB.01327-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunescu A, Garric J, Vollat B, Canet-Soulas E, Graveron-Demilly D, Fauvelle F. In vivo proton HR-MAS NMR metabolic profile of the freshwater cladoceran Daphnia magna. Mol BioSyst. 2010;6:121–125. doi: 10.1039/b915417e. [DOI] [PubMed] [Google Scholar]
- Catala A. Five Decades with Polyunsaturated Fatty Acids: Chemical Synthesis, Enzymatic Formation, Lipid Peroxidation and Its Biological Effects. J Lipids. 2013:Article ID 710290, 19. doi: 10.1155/2013/710290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wang Y, Qian Y, Zhao X, Wang Q. The synergistic toxicity of the multiple chemical mixtures: implications for risk assessment in the terrestrial environment. Environ Int. 2015;77:95–105. doi: 10.1016/j.envint.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, Frohlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak R, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, Moore DD. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci U S A. 2009;106:18831–18836. doi: 10.1073/pnas.0909731106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Crawford DL, Oleksiak MF. Effects of Anthropogenic Pollution on the Oxidative Phosphorylation Pathway of Hepatocytes from Natural Populations of Fundulus heteroclitus. Aquat Toxicol. 2015;165:231–240. doi: 10.1016/j.aquatox.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Finn RD, Henderson CJ, Scott CL, Wolf CR. Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway. Biochem J. 2009;417:43–54. doi: 10.1042/BJ20080740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, Zhuang DZ, Bell SA, Lu N, McKee M, Seed B, Freeman MW. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48:621–632. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- Gao J, Xie W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol Sci. 2012;33:552–558. doi: 10.1016/j.tips.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginjupalli GK, Baldwin WS. The time- and age-dependent effects of the juvenile hormone analog pesticide, pyriproxyfen on Daphnia magna reproduction. Chemosphere. 2013;92:1260–1266. doi: 10.1016/j.chemosphere.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginjupalli GK, Gerard PD, Baldwin WS. Arachidonic acid enhances reproduction in Daphnia magna and mitigates changes in sex ratios induced by pyriproxyfen. Environ Toxicol Chem. 2015;34:527–535. doi: 10.1002/etc.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrochategui E, Casas J, Pérez-Albaladejo E, Jáuregui O, Porte C, Lacorte S. Characterization of complex lipid mixtures in contaminant exposed JEG-3 cells using liquid chromatography and high-resolution mass spectrometry. Environ Sci Pollut Res Int. 2014;21:11907–11916. doi: 10.1007/s11356-014-3172-5. [DOI] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS. Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr Pharmacog Personal Med. 2009;7:81–105. doi: 10.2174/187569209788654005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MA, Pardee K, Liu S, King-Jones K, Lajoie G, Edwards A, Krause HM, Thummel CS. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev. 2009;23:2711–2716. doi: 10.1101/gad.1833609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ, Turner N, Storlien LH, Else PL. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc. 2005;80:155–169. doi: 10.1017/s1464793104006578. [DOI] [PubMed] [Google Scholar]
- Isaac G, Jeannotte R, Esch SW, Welti R. New mass-spectrometry-based strategies for lipids. Genet Eng (N Y) 2007;28:129–157. doi: 10.1007/978-0-387-34504-8_8. [DOI] [PubMed] [Google Scholar]
- Jordão R, Casas J, Fabrias G, Campos B, Piña B, Lemos MF, Soares AM, Tauler R, Barata C. Obesogens beyond vertebrates: lipid perturbation by tributyltin in the crustacean Daphnia magna. Environ Health Perspect. 2015;123:813–819. doi: 10.1289/ehp.1409163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimullina E, Li Y, Gingupalli GK, Baldwin WS. Daphnia HR96 is a promiscuous xenobiotic and endobiotic nuclear receptor. Aquat Toxicol. 2012;116–117:69–78. doi: 10.1016/j.aquatox.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T. Phosphatidylinositol-phosphatidic acid exchange by Nir2 at ER-PM contact sites maintains phosphoinositide signaling competence. Dev Cell. 2015;33:549–561. doi: 10.1016/j.devcel.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K, Horner MA, Lam G, Thummel CS. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006;4:37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Konno Y, Negishi M, Kodama S. The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet. 2008;23:8–13. doi: 10.2133/dmpk.23.8. [DOI] [PubMed] [Google Scholar]
- Koskinen H, Pehkonen P, Vehniainen E, Krasnov A, Rexroad C, Afanasyev S, Molsa H, Oikari A. Response of rainbow trout transcriptome to model chemical contaminants. Biochem Biophys Res Commun. 2004;320:745–753. doi: 10.1016/j.bbrc.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Lappas M, Mundra PA, Wong G, Huynh K, Jinks D, Georgiou HM, Permezel M, Meikle PJ. The prediction of type 2 diabetes in women with previous gestational diabetes mellitus using lipidomics. Diabetologia. 2015;58:1436–1442. doi: 10.1007/s00125-015-3587-7. [DOI] [PubMed] [Google Scholar]
- Latta LC, IV, Frederick S, Pfrender ME. Diet restriction and life history trade-offs in short- and long-lived species of Daphnia. J Exp Zool. 2011;315:610–617. doi: 10.1002/jez.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão BC, Rocha-Frigoni NA, Cabral EC, Coelho MB, Ferreira CR, Eberlin MN, Accorsi MF, Nogueira É, Mingoti GZ. Improved embryonic cryosurvival observed after in vitro supplementation with conjugated linoleic acid is related to changes in the membrane lipid profile. Theriogenology. 2015;84:127–136. doi: 10.1016/j.theriogenology.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Li CC, Lii CK, Liu KL, Yang JJ, Chen HW. DHA down-regulates phenobarbital-induced cytochrome P450 2B1 gene expression in rat primary hepatocytes by attenuating CAR translocation. Toxicol Appl Pharmacol. 2007;225:329–336. doi: 10.1016/j.taap.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Leo MA, Aleynik SI, Aleynik MK, DeCarli LM. Polyenylphosphatidylcholine decreases alcohol-induced oxidative stress in the baboon. Alcohol Clin Exp Res. 1997;21:375–379. [PubMed] [Google Scholar]
- Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, Lee KU, Pak YK, Lee HK. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litoff EJ, Garriott TE, Ginjupalli GK, Butler L, Gay C, Scott K, Baldwin WS. Annotation of the Daphnia magna nuclear receptors: Comparison to Daphnia pulex. Gene. 2014;552:116–125. doi: 10.1016/j.gene.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Li CC, Liu KL, Lii CK, Chen HW. Docosahexaenoic acid downregulates phenobarbital-induced cytochrome P450 2B1 gene expression in rat primary hepatocytes via the c-Jun NH2-terminal kinase mitogen-activated protein kinase pathway. Mol Nutr Food Res. 2008 doi: 10.1002/mnfr.200800112. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lu P, Yan J, Liu K, Garbacz WG, Wang P, Xu M, Ma X, Xie W. Activation of aryl hydrocarbon receptor dissociates fatty liver from insulin resistance by inducing fibroblast growth factor 21. Hepatology. 2015;61:1908–1919. doi: 10.1002/hep.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 2004;279:19832–19838. doi: 10.1074/jbc.M313601200. [DOI] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, Sueyoshi T. Nuclear pregnane X receptor crosstalk with FOXA2 to mediate the drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem. 2007;282:9768–9776. doi: 10.1074/jbc.M610072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead AW, LeBlanc GA. Insecticidal juvenile hormone analogs stimulate the production of male offspring in the crustacean Daphnia magna. Environ Health Perspect. 2003;111:919–924. doi: 10.1289/ehp.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma P, Palma VL, Matos C, Fernandes RM, Bohn A, Soares AM, Barbosa IR. Effects of atrazine and endosulfan sulphate on the ecdysteroid system of Daphnia magna. Chemosphere. 2009;74:676–681. doi: 10.1016/j.chemosphere.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Pape-Lindstrom PA, Lydy MJ. Synergistic toxicity of atrazine and organophosphate insecticides contravenes the response addition mixture model. Environ Toxicol Chem. 1997;16:2415–2420. [Google Scholar]
- Pavlaki MD, Ferreira AL, Soares AM, Loureiro S. Changes of chemical chronic toxicity to Daphnia magna under different food regimes. Ecotoxicol Environ Saf. 2014;109:48–55. doi: 10.1016/j.ecoenv.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Peng Y, Luo Y, Nie XP, Liao W, Yang YF, Ying GG. Toxic effects of triclosan on the detoxification system and breeding of Daphnia magna. Ecotoxicology. 2013;22:1384–1394. doi: 10.1007/s10646-013-1124-3. [DOI] [PubMed] [Google Scholar]
- Pieters BJ, Jager T, Kraak MH, Admiraal W. Modeling responses of Daphnia magna to pesticide pulse exposure under varying food conditions: intrinsic versus apparent sensitivity. Ecotoxicology. 2006;15:601–608. doi: 10.1007/s10646-006-0100-6. [DOI] [PubMed] [Google Scholar]
- Roling JA, Bain LJ, Gardea-Torresdey J, Bader J, Baldwin WS. Hexavalent chromium reduces juvenile growth and alters gene expression in Fundulus heteroclitus. Environ Toxicol Chem. 2006;25:62–70. doi: 10.1897/05-659r.1. [DOI] [PubMed] [Google Scholar]
- Sadowski MC, Pouwer RH, Gunter JH, Lubik AA, Quinn RJ, Nelson CC. The fatty acid synthase inhibitor triclosan: repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget. 2014;5:9362–9381. doi: 10.18632/oncotarget.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O, Santo N, Tohkin M, Furukawa Y, Gonzalez FJ, Komai M. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol Appl Pharmacol. 2008;229:10–19. doi: 10.1016/j.taap.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Scanlan LD, Loguinov AV, Teng Q, Antczak P, Dailey KP, Nowinski DT, Kornbluh J, Lin XX, Lachenauer E, Arai A, Douglas NK, Falciani F, Stapleton HM, Vulpe CD. Gene expression, metabolite and lipid profiling in eco-indicator Daphnia magna indicate diverse mechanisms of toxicity by legacy and emerging flame-retardants. Environ Sci Technol. 2015;49:7400–7410. doi: 10.1021/acs.est.5b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechtriem C, Arts MT, Zellmer ID. Effect of temperature on the fatty acid composition and temporal trajectories of fatty acids in fasting Daphnia pulex (Crustacea, Cladocera) Lipids. 2006;41:397–400. doi: 10.1007/s11745-006-5111-9. [DOI] [PubMed] [Google Scholar]
- Sengupta N, Litoff EJ, Baldwin WS. The HR96 activator, atrazine, reduces sensitivity of D. magna to triclosan and DHA. Chemosphere. 2015;128:299–306. doi: 10.1016/j.chemosphere.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Thummel CS. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab. 2009;10:481–490. doi: 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Thummel CS. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab. 2012;15:122–127. doi: 10.1016/j.cmet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale SJ, Kainz MJ, Brett MT. Diet-switching experiments show rapid accumulation and preferential retention of highly unsaturated fatty acids in Daphnia. Oikos. 2011;120:1674–1682. [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Uhl O, Demmelmair H, Segura MT, Florido J, Rueda R, Campoy C, Koletzko B. Effects of obesity and gestational diabetes mellitus on placental phospholipids. Diabetes Res Clin Pract. 2015;109:364–371. doi: 10.1016/j.diabres.2015.05.032. [DOI] [PubMed] [Google Scholar]
- Verreth J, Coppoolse J, Segner H. The effect of low HUFA- and high HUFA-enriched Artemia, fed at different feeding levels, on growth, survival, tissue fatty acids and liver histology of Clarias gariepinus larvae. Aquaculture. 1994;126:137–150. [Google Scholar]
- Wei D, Li J, Shen M, Jia W, Chen N, Chen T, Su D, Tian H, Zheng S, Dai Y, Zhao A. Cellular production of n-3 PUFAs and reduction of n-6-to n-3 ratios in the pancreatic b-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes. 2010;59:471–478. doi: 10.2337/db09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbecher C, Mühlenbruch K, Kröger J, Jacobs S, Kuxhaus O, Floegel A, Fritsche A, Pischon T, Prehn C, Adamski J, Joost HG, Boeing H, Schulze MB. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am J Clin Nutr. 2015;101:1241–1250. doi: 10.3945/ajcn.114.099150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.