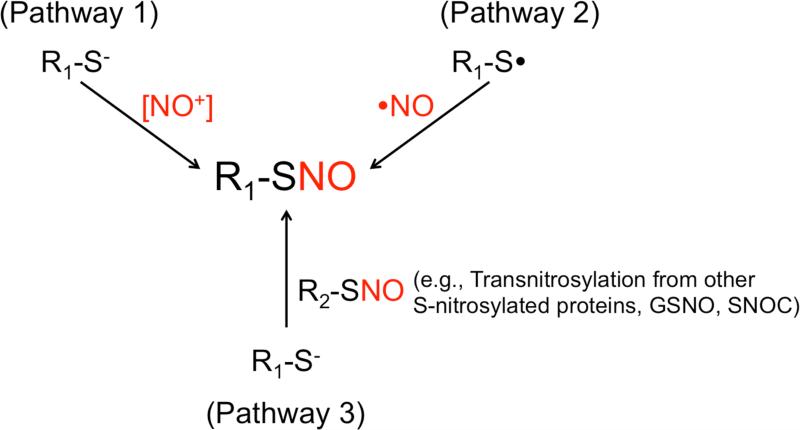
Fig. 1.
Possible mechanisms by which cysteine thiol residues form S-nitrosothiols in vivo. (Pathway 1) As an active intermediate, nitrosonium cation [NO+], with brackets indicated the reaction intermediate rather than free NO+, may be generated from a metal ion-dependent oxidation of NO. [NO+] reacts with a thiolate anion (R-S−) to form an S-nitrosothiol (R-SNO). (Pathway 2) Radical recombination of •NO with thiyl radical (RS•) yields an S-nitrosothiol. (Pathway 3) Transnitrosylation from an S-nitrosothiol (R2-SNO), such as another S-nitrosylated protein, GSNO or SNOC. Here, the NO group is transferred from one thiol to another.