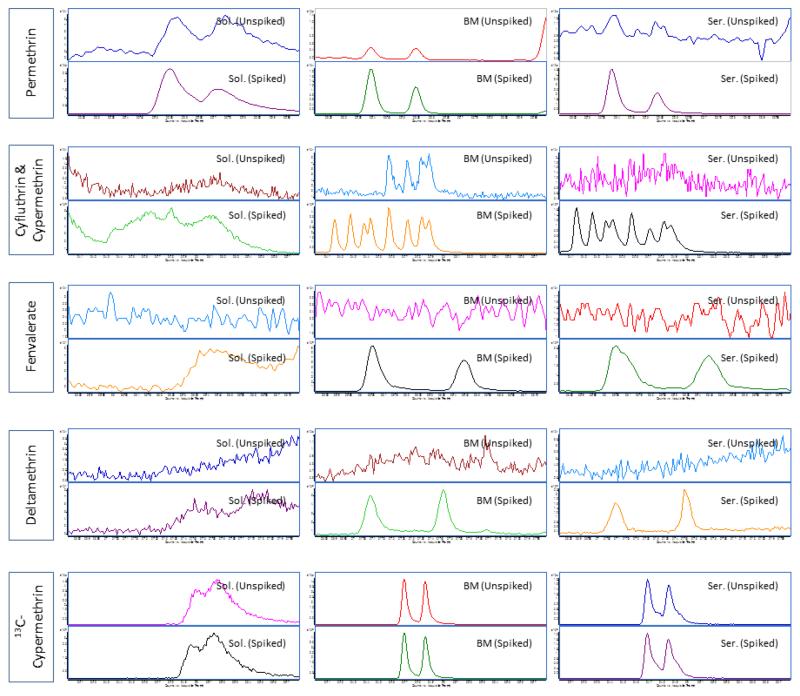
Figure 2. An example of matrix-induced chromatographic response observed for pyrethroids when analyzed in breast milk and serum samples.
Note: Pyrethroids in serum and breast milk samples were analyzed using a GC-MS/MS system (7000) from Agilent Technologies(Waldbronn, Germany) coupled with electron impact ionization interface. The GC and MS modules were programmed and controlled using Mass Hunter Software version B.03.01 (Agilent Technologies). A DB-5MS (30 m × 0.250 mm ID × 0.50 μm film thickness) analytical column from Agilent Technologies was used. Serum samples were subjected to protein precipitation using a mixture of water and propanol (85:15 v/v) prior to extraction with C18 solid phase extraction cartridges (6 cc, 500 mg) (JT Baker, Center Valley, PA). The eluents (in hexane/ethyl ether) were further purified using florisil cartridges (6 cc, 500 mg) (Restek Corp, Bellefonte, CA) while breast milk samples were subjected to liquid-liquid extraction (using acetonitrile followed by hexane) and overnight freezing to remove lipids. Extractants were then loaded onto ENVI-Carb-II™/PSA cartridges (6cc, 500/300 mg) (Sigma Aldrich, St. Louis, MO). Final eluents (from both methods) were evaporated to dryness and the residues were reconstituted with 50 μL of an acetonitrile-toluene mixture (66:34 v/v) prior to injection. Theinjection volume was 2 μL under pulsed splitless mode and an injector temperature of 250 °C. A multi-stepwise gradient temperature program (from 70 °C to 310 °C) was used during chromatographic separation. The total run time was 40.2 min. The flow rate of carrier gas helium was at 1.2 mL/min. Quantitative MS/MS transitions were: permethrin m/z183.2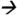 77.0@40V CE; cypermethrin and cyfluthrin m/z163.1
77.0@40V CE; cypermethrin and cyfluthrin m/z163.1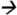 127.2@5V CE; fenvalerate m/z125.2
127.2@5V CE; fenvalerate m/z125.2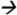 89.0@20V CE; deltamethrin m/z253.1
89.0@20V CE; deltamethrin m/z253.1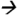 93.2@20V CE; and cypermethrin (13C) m/z170.0
93.2@20V CE; and cypermethrin (13C) m/z170.0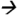 98.0@15V CE.
98.0@15V CE.