Abstract
Objective
This study was conducted using the human papillomavirus (HPV) DNA chip test (HDC), in order to determine whether the HPV genotype is a predictor of residual disease in a subsequent hysterectomy following a loop electrosurgical excision procedure (LEEP) for cervical intraepithelial neoplasia (CIN) 3.
Methods
Between January 2002 and February 2015, a total of 189 patients who underwent a hysterectomy within 6 months of LEEP caused by CIN 3 were included in this study. We analyzed their epidemiological data, pathological parameters, high-risk HPV (HR-HPV) load as measured by the hybrid capture II assay, and HR-HPV genotype as measured by the HDC. A logistic regression model was used to analyze the relationship between covariates and the probability of residual disease in subsequent hysterectomy specimens.
Results
Of the 189 patients, 92 (48.7%) had residual disease in the hysterectomy specimen, CIN 2 in seven patients, CIN 3 in 79 patients, IA1 cancer in five patients, and IA2 cancer in one patient. Using multivariate analysis, the results were as follows: cone margin positivity (odds ratio [OR], 2.43; 95% CI, 1.18 to 5.29; p<0.05), HPV viral load ≥220 relative light unit (OR, 2.98; 95% CI, 1.38 to 6.43; p<0.01), positive endocervical cytology (OR, 8.97; 95% CI, 3.81 to 21.13; p<0.001), and HPV-16 or HPV-18 positivity (OR, 9.07; 95% CI, 3.86 to 21.30; p<0.001).
Conclusion
The HPV-16 or HPV-18 genotype is a reliable predictive factor of residual disease in a subsequent hysterectomy following a LEEP for CIN 3.
Keywords: Cervical Intraepithelial Neoplasia; Conization; Human Papillomavirus; Hysterectomy; Neoplasm, Residual
INTRODUCTION
Conization by a loop electrosurgical excision procedure (LEEP) is considered an appropriate treatment for cervical intraepithelial neoplasia (CIN) 3. The majority of CIN 1 lesions regress spontaneously, however less frequently, they may progress to CIN 2-3 [1,2]. Once a lesion progresses to CIN 3, the rate of progression to invasive cancer dramatically increases, up to 12% [3], with an approximate 100% progression rate if the observation period is long enough [4].
Residual disease after incomplete excision by conization of CIN 3 is found in 29% to 57% of patients who subsequently undergo a hysterectomy [5,6]. Therefore, accurate prediction of residual disease following conization is of utmost importance for the conservative treatment and counseling of patients with CIN 3. Without data regarding accurate prediction of residual disease after conization, physicians are left with difficulty in counseling patients with CIN 3 who desire definitive answers about the most appropriate next step.
Several clinicopathological factors including age, parity, menopausal status, cone margin status, and post-LEEP endocervical cytology, have been reported to be predictive of residual disease after conization [7]. With respect to human papillomavirus (HPV) DNA testing, high-risk HPV (HR-HPV) viral load as measured using the hybrid capture II assay (HC2; Digene Co., Gaithersburg, MD, USA) prior to conization has been evaluated as a predictor of residual disease in subsequent hysterectomy specimens [6]. In addition, a pre-hysterectomy HPV test has been proposed as a possible predictor of residual disease [6,8]. However, the relationship between the HPV genotype and residual disease in a subsequent hysterectomy following conization is not yet established.
The aim of this study therefore, was to determine whether the HPV genotype is a predictor of residual disease in a subsequent hysterectomy following a LEEP for CIN 3.
MATERIALS AND METHODS
We retrospectively reviewed the records of all 2,571 patients with histologically-confirmed CIN 3, who had been treated by a LEEP at the Department of Obstetrics and Gynecology of Chonnam National University Hospital (CNUH) between January 2002 and February 2015.
One hundred eighty-nine patients were considered eligible for the current study if they fulfilled the following criteria: (1) histologically confirmed CIN 3 by a LEEP; (2) patients in whom both pre-LEEP HR-HPV test results from the HPV DNA chip test (HDC; MyGene Co., Seoul, Korea) and the HC2 were available; and (3) patients who underwent a hysterectomy within 6 months of LEEP. Epidemiological data, HR-HPV test data from the HDC and HC2, and pathological data were obtained from the medical records.
The LEEP was performed by one of the two gynecologic oncologists (SMK and WDK). The cervix was exposed using an adapted speculum allowing smoke evacuation. After the cervix was swabbed with Lugol's iodine solution to assist in locating the ectocervical margins of the lesion, 1 mL of vasoconstrictors was injected into each quadrant. The loop was selected according to the size of the area to be excised among 1×2 cm (width×length) sized loop, 1.5×1.5 cm (width×length) sized loop, and 2×2 cm (width×length) sized loop. When possible, the cervix lesion was excised en bloc for better orientation and margin status interpretation. When the exocervical lesion was too large to be accommodated by a single sweep, it was excised with two or more systematic sweeps, and the pieces of specimens were reassembled to original anatomic shape by the operator before sending it to pathology room to make the pathologists indicate the true excisional margins in specimens. The endocervical sweep was routinely performed using 1×2 cm (width×length) sized loop. A section was placed at the 12 o'clock position in the LEEP specimen for orientation, and the specimens were subsequently fixed in 10% formalin for pathological examination.
All patients who underwent a hysterectomy after a LEEP did so though an abdominal, vaginal, or laparoscopic route as indicated, based on the preference of the attending physicians and the indication for hysterectomy. The latter included 134 patients with positive cone margins or positive endocervical cytology obtained immediately after a LEEP (endocervical cytology), 44 patients with gynecological disease (uterine myoma or adenomyosis), six patients with poor compliance with follow-up, and five patients with cancer phobia. For statistical analysis, residual disease was defined as the presence of CIN 2, CIN 3, or invasive cancer in the hysterectomy specimens.
The study protocol was evaluated and approved by the Institutional Review Board at CNUH.
1. Hybrid capture II assay
The samples were collected by placing a cytobrush into the exocervix and rotating the brush 3 times. The sample was kept frozen at −20°C in a collection tube (Digene Co.) until needed. The denatured single-strand DNA was hybridized with a RNA researcher of the mixed HR-HPV group. This reaction mixture was placed in microtiter wells coated with primary antibodies for the RNA/DNA hybrid. Following RNA/DNA hybrid-antibody bonding, the mixture was incubated with alkaline phosphatase-conjugated secondary antibodies, washed, and lumi-Phospho 530 was added, to react with the dioxetane-based chemiluminescent substrate. Alkaline phosphatase was added to obtain luminescence, which was measured using a luminometer and expressed in relative light units (RLUs). The solution containing 1 pg/mL HPV-16 DNA was used as a positive control for the HR-HPV group. The RLUs for all samples were set to the degree of relative brightness in comparison to the positive control. This ratio was considered positive at a value of 1.0 or greater, and negative at a value of 1.0 or less. The samples were analyzed for the presence of the 13 types of HR-HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68).
2. HDC test
We used the HDC, a PCR-based DNA microarray system, as a HPV genotyping method. The HDC contains 24 type-specific probes; 15 probes are HR types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) and 9 probes are low-risk types (6, 11, 34, 40, 42, 43, 44, 54, and 70). Briefly, DNA was isolated from a swab sample using a DNA isolation kit (MyGene Co.), and target L1 regions of HPV DNA were subsequently amplified and labeled using a single dye (indocarbocyanine-dUTP; NEN Life Science Products Inc., Boston, MA, USA). The PCR products of all samples were detected using electrophoresis with a 2.5% agarose gel. The samples were mixed with a hybridization solution (MyGene Co.), and hybridization was performed at 43°C for 90 minutes. The hybridized HPV DNA was visualized using a DNA chip scanner (Scanarray Lite, GSI Lumonics, Ottawa, ON, Canada). Fifteen types of HR-HPV positivity were used to assess the HDC performance.
3. Statistical analysis
A statistical comparison was carried out using a Student t-test or a Fisher exact test. Agreement between tests was assessed by Cohen's κ statistic, and the p-value was calculated using McNemar's test, with values between 0.00 and 0.20 indicating poor agreement, values between 0.21 and 0.40 indicating fair agreement, values between 0.41 and 0.60 indicating moderate agreement, values between 0.61 and 0.80 indicating substantial agreement, and values between 0.81 and 1.00 indicating near-perfect agreement. A receiver operating characteristic (ROC) curve was used to determine the clinically most useful cutoff value of HR-HPV viral load for predicting residual disease. A logistic regression model was used to analyze the relationship between covariates and the probability of residual disease in subsequent hysterectomy samples. The data were analyzed using the SPSS ver. 21.0 (IBM Co., Armonk, NY, USA). The 95% CIs were calculated. All p-values reported are two-sided, and p-values <0.05 were considered statistically significant.
RESULTS
Of the 189 patients, 92 (48.7%) had residual disease in their hysterectomy specimen. The residual disease was found to be CIN 2 in seven patients, CIN 3 in 79 patients, IA1 cancer in five patients, and IA2 cancer in one patient. The mean lag time between the LEEP and the hysterectomy was 31.7 days (range, 9 to 171 days). The HPV detection rate of the HDC was almost comparable to that of the HC2, being 185 patients (97.9%) and 184 patients (97.3%), respectively.
The characteristics of the 189 eligible patients are listed in Table 1. The mean age of the patients was 53.8 years old (range, 44 to 83 years), 95 patients (50.3%) were postmenopausal, 126 patients had cone margin involvement, and 71 patients had positive endocervical cytology. The median HPV viral load of all patients was 180.9 RLU (range, 0.1 to 2,726.0 RLU). Patients with residual disease did not differ from non-residual patients with respect to age, parity, menopause, and HR-HPV status at the time of the LEEP. Cone margin involvement, positive endocervical cytology, a high HPV viral load as measured by the HC2, and HPV positivity to HPV-16 or HPV-18 were associated with a significantly higher risk of residual disease. The median values of HPV viral load were 158.1 RLU (range, 0.1 to 2,726.0 RLU) for non-residual patients and 500.5 RLU (range, 2.4 to 2,289.4 RLU) for residual patients (p<0.01).
Table 1. Patient characteristics.
| Characteristic | Residual disease in hysterectomy specimen | p-value | |
|---|---|---|---|
| Absent (n=97) | Present (n=92) | ||
| Age (yr) | 0.07 | ||
| Mean±SD | 53.1±7.4 | 54.6±8.7 | |
| Range | 45-77 | 44-83 | |
| Parity | 0.19 | ||
| Mean±SD | 2.5±1.0 | 2.8±1.3 | |
| Range | 0-6 | 0-6 | |
| Menopause | 0.72 | ||
| No | 50 | 44 | |
| Yes | 47 | 48 | |
| HC2 | 0.06 | ||
| Negative | 5 | 0 | |
| Positive | 92 | 92 | |
| HDC | 0.13 | ||
| Negative | 4 | 0 | |
| Positive | 93 | 92 | |
| Cone margin status | <0.01 | ||
| Negative | 45 | 18 | |
| Positive | 52 | 74 | |
| Endocervical cytology | <0.01 | ||
| Negative | 83 | 35 | |
| Positive | 14 | 57 | |
| HPV viral load by HC2 | <0.01 | ||
| Median | 158.1 | 500.5 | |
| Range | 0.1-2,726.0 | 2.4-2,289.4 | |
| HPV-16 or HPV-18 by HDC | <0.01 | ||
| Negative | 57 | 17 | |
| Positive | 40 | 75 | |
HC2, hybrid capture II test; HDC, HPV DNA chip test; HPV, human papillomavirus.
The concordant and discordant results between the 2 HPV tests are summarized in Table 2. The overall agreement between the 2 tests was 99.5%, with a κ value of 0.886. All 184 HR-HPV-positive specimens, as measured by the HC2, were also positive on the HDC. Among the five patients who measured negative with the HC2, the HDC was also negative in 4 (80.0%). When the HDC was compared with the HC2, discordant results were only observed in one patient, showing HC2-negative/HDC-positive results. Genotyping of this 1 HC2-negative/HDC-positive case revealed the presence of HPV-53.
Table 2. The level of concordance between HR-HPV tests.
| HC2* | No. of specimens (%) with HDC* | Total no. of specimens (%) | |
|---|---|---|---|
| Negative | Positive | ||
| Negative | 4 | 1 | 5 (3.4) |
| Positive | 0 | 184 | 184 (96.6) |
| Total | 4 (2.4) | 185 (97.6) | |
HC2, hybrid capture II test; HDC, HPV DNA chip test; HPV, human papillomavirus; HR, high risk.
*Absolute agreement=99.5%, κ=0.886 (p<0.001). Agreement between tests was assessed by Cohen's κ statistic. A p-value was calculated using McNemar's test.
The distribution of prevalent HR-HPV genotypes in residual disease as measured by the HDC is presented in Table 3. Of the 185 patients with HPV infection, 161 tested positive for a single type of HPV, and 24 tested positive for multiple types of HPV. There were no significant differences between the residual and non-residual groups with respect to infection with single or multiple types of HPV (p=0.81). Among the 92 patients with residual disease, a single HR-HPV infection was positive in 85.2%. HPV-16 (33.3%) was the most prevalent single HR-HPV infection type, with the next most common types being HPV-18 (16.4%), HPV-52 (9.5%), and HPV-58 (8.5%). Of the 92 patients with residual disease, 75 patients (81.5%) had positivity to HPV-16 or HPV-18. HPV-16 and HPV-18 were associated with a significantly higher risk of residual disease than the other HPV genotypes (respective p=0.02 and p<0.001).
Table 3. The correlation of HR-HPV genotypes by HDC and residual disease.
| Variable | Residual disease in hysterectomy specimen | Total (n=189) | |
|---|---|---|---|
| Absent (n=97) | Present (n=92) | ||
| None (n=4) | 4 | 0 | 4 |
| Single infection (n=161) | |||
| 16* | 24 | 39 | 63 |
| 18* | 7 | 24 | 31 |
| 52 | 12 | 6 | 18 |
| 58 | 11 | 5 | 16 |
| 31 | 5 | 3 | 8 |
| 33 | 2 | 1 | 3 |
| 56 | 1 | 1 | 2 |
| Other types† | 20 | 0 | 20 |
| Multiple infection (n=24) | |||
| 16+18 | 3 | 5 | 8 |
| 16+58 | 1 | 3 | 4 |
| 18+58 | 1 | 3 | 4 |
| 16+52 | 0 | 1 | 1 |
| 39+56 | 0 | 1 | 1 |
| Other mixed types | 6 | 0 | 6 |
HDC, HPV DNA chip test; HPV, human papillomavirus; HR, high risk.
*Significantly higher than the results for other HR-HPV genotype infection (chi-square test; p<0.05). †High-risk human papillomavirus types 35, 39, 45, 51, 53, 59, 66, and 68.
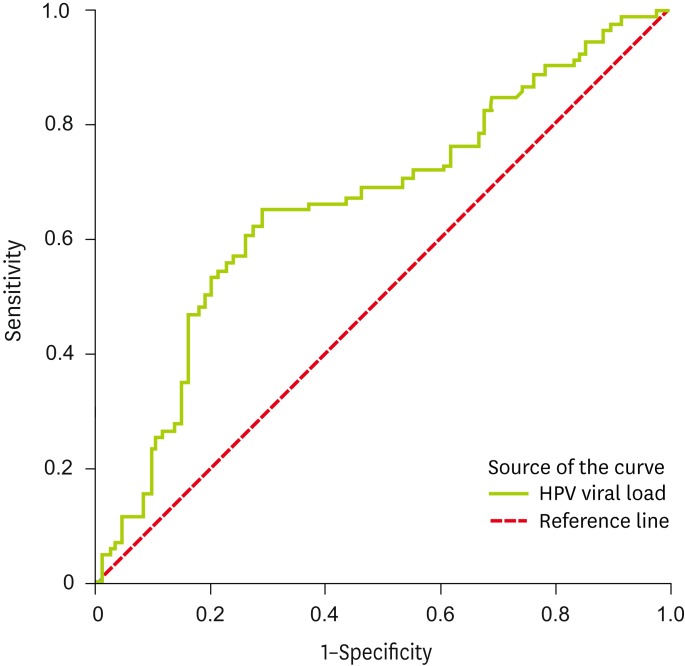
Our data produced the ROC curve shown in Fig. 1. The ROC curve was used to determine the most useful HPV viral load level in the prediction of residual disease in a subsequent hysterectomy following a LEEP for CIN 3. The area under a ROC curve (area under the curve, AUC), with a value of 1.0, corresponds to a 100% accurate prediction of the outcome, while an AUC of 0.5 equates to a toss of a coin probability of the correct prediction being made. In the present study, the point on the curve closest to the upper left corner corresponds to a threshold HPV viral load level of 220 RLU. The AUC for HPV viral load was 0.663 (p<0.01). At this cut-off level, the HPV viral load was able to predict residual disease with a sensitivity of 65.2%, a specificity of 70.1%, and an accuracy of 67.7%.
Fig. 1.
Receiver operating characteristic curve for human papillomavirus (HPV) viral load.
In the multivariate logistic regression model, the odds ratio (OR) was adjusted for covariates. According to this analysis, the independent factors that are significantly predictive of residual disease following conization were cone margin positivity (OR, 2.43; 95% CI, 1.18 to 5.29; p<0.05), HPV viral load ≥220 RLU (OR, 2.98; 95% CI, 1.38 to 6.43; p<0.01), positive endocervical cytology (OR, 8.97; 95% CI, 3.81 to 21.13; p<0.001), and HPV-16 or HPV-18 positivity (OR, 9.07; 95% CI, 3.86 to 21.30; p<0.001) (Table 4).
Table 4. Factor predicting residual disease in subsequent hysterectomy specimen.
| Factor | Residual disease in hysterectomy specimen | Multivariate analysis | ||
|---|---|---|---|---|
| Absent (n=97) | Present (n=92) | OR (95% CI) | p-value | |
| Cone margin status | ||||
| Negative | 45 (71.4) | 18 (28.6) | 1 | |
| Positive | 52 (41.3) | 74 (58.7) | 2.43 (1.18-5.29) | <0.05 |
| HPV viral load by HC2 | ||||
| <220 RLU/PC | 68 (68.0) | 32 (32.0) | 1 | |
| ≥220 RLU/PC | 29 (32.6) | 60 (67.4) | 2.98 (1.38-6.43) | <0.01 |
| Endocervical cytology | ||||
| Negative | 83 (70.3) | 35 (29.7) | 1 | |
| Positive | 14 (19.7) | 57 (80.3) | 8.97 (3.81-21.13) | <0.001 |
| HPV-16 or HPV-18 by HDC | ||||
| Negative | 57 (77.0) | 17 (23.0) | 1 | |
| Positive | 40 (34.8) | 75 (65.2) | 9.07 (3.86-21.30) | <0.001 |
Values are presented as number (%).
CI, confidence interval; HC2, hybrid capture II test; HDC, HPV DNA chip test; HPV, human papillomavirus; OR, odds ratio; PC, positive controls; RLU, relative light unit.
DISCUSSION
Conservative treatment with a LEEP is both a diagnostic and therapeutic procedure that can effectively eradicate CIN 3 at every stage of a woman's life [9], and it is important to avoid any residual disease in the remaining cervix following a LEEP. Therefore, the prediction of the probability of post-LEEP residual disease in patients with CIN 3 is important for patient counseling and management.
Although the cone margin status of LEEP specimens has been proposed as an accurate predictive factor for residual disease after a LEEP for CIN 3 [9], a free margin does not always indicate complete excision, due to the possibility of multifocal lesions or inadequate specimen tissue resulting from ablative conization. Residual disease can be found in up to 23% to 31% of patients with cone margin negativity [10,11], and in addition, it is not found in up to 37% to 60% of patients with cone margin positivity [6,10]. Therefore, the identification of patients for a hysterectomy based on cone margin status alone would likely result in the over-treatment or under-treatment of many women. In our series, 41.3% of patients were over-treated and 28.6% were under-treated based on cone margin status. These findings are similar to those shown in previous reports [6,10,11]; therefore, it is clear that more accurate predictive factors are required.
Endocervical cytology obtained immediately after a LEEP was identified as a highly significant pathological parameter to predict residual disease in subsequent hysterectomy specimens [10,12,13]. The residual disease rate was 74% to 89% when endocervical cytology was positive, compared with 25% to 26% of residual disease when endocervical cytology was negative [12,13]. Moore et al. [10] highlighted that positive endocervical cytology was significantly associated with residual disease (OR, 2.1). Schermerhorn et al. [14] reported that 8 out of 12 patients (67%) with both positive cone margin and endocervical cytology had residual dysplasia compared with 3 out of 17 patients (18%) with a negative cone margin and positive endocervical cytology. Kobak et al. [15] emphasized the need for routine post-cone endocervical cytology, whether for the prediction of post-cone residual disease or for the diagnosis of occult invasive cancer. Our results show that positive endocervical cytology was an independent predictive factor for residual disease (OR, 8.97; 95% CI, 3.81 to 21.13; p<0.001). The sensitivity, specificity, and accuracy of positive endocervical cytology in predicting residual disease were 62.0%, 85.6%, and 74.1%, respectively.
Little is known about the use of the HR-HPV test in predicting residual disease in a subsequent hysterectomy following conization [6]. It has been reported that patients were arbitrarily divided according to the median HPV viral load of the total patients, and that a HPV viral load ≥300 RLU was a predictive factor for residual disease (OR, 2.96; 95% CI, 1.1 to 8.1; p=0.034). In the current study, a ROC curve was used to determine the most useful HPV viral load level in predicting residual disease, and a HPV viral load level of 220 RLU was shown to have the most predictive power. The AUC for HPV viral load was 0.663 (p<0.01). At this cut-off level, in the multivariate analysis, a HPV viral load ≥220 RLU was found to be an accurate predictive factor for residual disease (OR, 2.98; 95% CI, 1.38 to 6.43; p<0.01).
The HDC is a newly developed biotechnology that may be applied to the detection and typing of HPV. By comparing the results of the HPV DNA sequencing using the same samples, the accuracy of the HDC in the detection and typing of HPV in cervical lesions was shown to be 257 out of 282 cases (91.1%) [16]. In the current study, the degree of concordance between the HDC and the HC2 was 99.5% (Cohen's κ, 0.886 [near-perfect agreement]), and the HPV detection rate determined by the HDC was comparable to that determined by the HC2. In one of our previous studies assessing the concordance between both HPV tests in 672 patients with CIN 2-3, the overall agreement between the 2 tests was 97.3%, with a κ value of 0.815 (near-perfect agreement) [17]. The HPV detection rate determined by the HDC was comparable to that determined by the HC2 in patients with CIN 2-3.
We have shown that the residual CIN 3 rate in women containing HPV-16 and HPV-18 at the time of LEEP is significantly higher than in women with other HPV genotypes. Our results are in line with previous findings that HPV-16 and HPV-18 exhibits a lower clearance rate than other HPV types and an increased CIN 2/3 risk [18]. Among the 92 patients with residual disease, 75 patients (81.5%) had positivity to HPV-16 or HPV-18 irrespective of coinfection. HPV-16 and HPV-18 were associated with a significantly higher risk of residual disease than other HPV genotypes (p=0.02 and p<0.001, respectively). In the multivariate analysis, a positive cone margin, HPV viral load ≥220 RLU, positive endocervical cytology, and positivity to HPV-16 or HPV-18 were predictive of residual disease. Of these significant parameters, positivity to HPV-16 or HPV-18 is a strong independent predictive factor of residual disease in a subsequent hysterectomy following a LEEP for CIN 3 (OR, 9.07; 95% CI, 3.86 to 21.30; p<0.001).
Although several studies have found that old age, high parity, and menopausal status were predictive of residual disease [7,10,13,19,20], other studies have reported no association [21]. Here, we found no significant association between the probability of residual disease and age, parity, or menopausal status.
To our knowledge, the current study is the first to analyze whether the HR-HPV genotype is predictive of residual disease in a subsequent hysterectomy following a LEEP for CIN 3. The most significant finding of this study is that positivity to HPV-16 or HPV-18 is an independent predictive factor of residual disease after a LEEP for CIN 3. The limitations of our study include the inherent biases of the retrospective nature of the design. We were unable to evaluate both the size of CIN 3 lesion (e.g., demonstrated by Lugol's solution) before LEEP and the matched volume of the resected specimen in the same patient for evaluating the appropriateness of LEEP. In addition, this study has the potential selection bias. There are approximate 30% of patients who underwent hysterectomy for benign indications; benign patients have a lower possibility of residual disease than those who have CIN 3 related indications for hysterectomy. Also, the follow-up period was relatively short to identify spontaneous regression and the HDC for verifying the HR-HPV genotype has not been approved by the Food and Drug Administration.
In conclusion, the HR-HPV genotype is a reliable prognostic marker of residual disease following a LEEP for CIN 3, and is more accurate than the HPV viral load, endocervical cytology, or cone margin status at the time of the LEEP. Although this finding requires substantiation in a further large-scale prospective investigation using standardized PCR-techniques for HPV detection, HPV-16 and HPV-18 should be considered as a risk factor for residual disease after a LEEP for CIN 3 and such patients warrant special attention with compulsive follow-up.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Falls RK. Spontaneous resolution rate of grade 1 cervical intraepithelial neoplasia in a private practice population. Am J Obstet Gynecol. 1999;181:278–282. doi: 10.1016/s0002-9378(99)70548-x. [DOI] [PubMed] [Google Scholar]
- 2.Nasiell K, Roger V, Nasiell M. Behavior of mild cervical dysplasia during long-term follow-up. Obstet Gynecol. 1986;67:665–669. doi: 10.1097/00006250-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Ostör AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–192. [PubMed] [Google Scholar]
- 4.Mitchell MF, Hittelman WN, Hong WK, Lotan R, Schottenfeld D. The natural history of cervical intraepithelial neoplasia: an argument for intermediate endpoint biomarkers. Cancer Epidemiol Biomarkers Prev. 1994;3:619–626. [PubMed] [Google Scholar]
- 5.Buxton EJ, Luesley DM, Wade-Evans T, Jordan JA. Residual disease after cone biopsy: completeness of excision and follow-up cytology as predictive factors. Obstet Gynecol. 1987;70:529–532. [PubMed] [Google Scholar]
- 6.Park JY, Lee SM, Yoo CW, Kang S, Park SY, Seo SS. Risk factors predicting residual disease in subsequent hysterectomy following conization for cervical intraepithelial neoplasia (CIN) III and microinvasive cervical cancer. Gynecol Oncol. 2007;107:39–44. doi: 10.1016/j.ygyno.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Lu CH, Liu FS, Tseng JJ, Ho ES. Predictive factors for residual disease in subsequent hysterectomy following conization for CIN III. Gynecol Oncol. 2000;79:284–288. doi: 10.1006/gyno.2000.5949. [DOI] [PubMed] [Google Scholar]
- 8.Lin CT, Tseng CJ, Lai CH, Hsueh S, Huang KG, Huang HJ, et al. Value of human papillomavirus deoxyribonucleic acid testing after conization in the prediction of residual disease in the subsequent hysterectomy specimen. Am J Obstet Gynecol. 2001;184:940–945. doi: 10.1067/mob.2001.112589. [DOI] [PubMed] [Google Scholar]
- 9.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 ASCCP Consensus Guidelines Conference 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 10.Moore BC, Higgins RV, Laurent SL, Marroum MC, Bellitt P. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361–366. doi: 10.1016/0002-9378(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 11.Phelps JY, 3rd, Ward JA, Szigeti J, 2nd, Bowland CH, Mayer AR. Cervical cone margins as a predictor for residual dysplasia in post-cone hysterectomy specimens. Obstet Gynecol. 1994;84:128–130. [PubMed] [Google Scholar]
- 12.Husseinzadeh N, Shbaro I, Wesseler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198–200. doi: 10.1016/0090-8258(89)90551-9. [DOI] [PubMed] [Google Scholar]
- 13.Kalogirou D, Antoniou G, Karakitsos P, Botsis D, Kalogirou O, Giannikos L. Predictive factors used to justify hysterectomy after loop conization: increasing age and severity of disease. Eur J Gynaecol Oncol. 1997;18:113–116. [PubMed] [Google Scholar]
- 14.Schermerhorn TJ, Hodge J, Saltzman AK, Hackett TE, Sprance HE, Harrison TA. Clinicopathologic variables predictive of residual dysplasia after cervical conization. J Reprod Med. 1997;42:189–192. [PubMed] [Google Scholar]
- 15.Kobak WH, Roman LD, Felix JC, Muderspach LI, Schlaerth JB, Morrow CP. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol. 1995;85:197–201. doi: 10.1016/0029-7844(94)00389-U. [DOI] [PubMed] [Google Scholar]
- 16.Choi YD, Jung WW, Nam JH, Choi HS, Park CS. Detection of HPV genotypes in cervical lesions by the HPV DNA Chip and sequencing. Gynecol Oncol. 2005;98:369–375. doi: 10.1016/j.ygyno.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Kang WD, Oh MJ, Kim SM, Nam JH, Park CS, Choi HS. Significance of human papillomavirus genotyping with high-grade cervical intraepithelial neoplasia treated by a loop electrosurgical excision procedure. Am J Obstet Gynecol. 2010;203:72.e1–72.e6. doi: 10.1016/j.ajog.2010.01.063. [DOI] [PubMed] [Google Scholar]
- 18.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Chang HY, Huang CC, Changchien CC. Prediction of disease persistence after conization for microinvasive cervical carcinoma and cervical intraepithelial neoplasia grade 3. Int J Gynecol Cancer. 2004;14:311–316. doi: 10.1111/j.1048-891x.2004.14215.x. [DOI] [PubMed] [Google Scholar]
- 20.Livasy CA, Maygarden SJ, Rajaratnam CT, Novotny DB. Predictors of recurrent dysplasia after a cervical loop electrocautery excision procedure for CIN-3: a study of margin, endocervical gland, and quadrant involvement. Mod Pathol. 1999;12:233–238. [PubMed] [Google Scholar]
- 21.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Human papillomavirus test after conization in predicting residual disease in subsequent hysterectomy specimens. Obstet Gynecol. 2009;114:87–92. doi: 10.1097/AOG.0b013e3181ab6dca. [DOI] [PubMed] [Google Scholar]