Abstract
AIM: To prepare valaciclovir polybutylcyan-oacrylate nanopa rticles (VACV-PBCA-NP) with liver targeting and hepatocyte permeable characteristics.
METHODS: Emulsion polymerization method was employed to prepare VACV-PBCA-NP. The formula and preparation conditions were optimized by using the uniform design. The organ distribution of the intravenously injected VACV-P BCA-NP and VACV in animal was determined using HPLC. The hepatocytes permeability of VACV-PBCA-NP was demonstrated by cell uptake experiment in vitro.
RESULTS: The drug loading and the drug embedding ratio of VACV-PBCA-NP were 11.20% and 84.85% respectively, with an average diameter of 104.77 nm ± 11.78 nm. The releasing characteristics in vitro fitted the two-phase kinetics. 74.49% of the drug was found to localize in the liver 15 min after the administration of VACV-PBCA-NP in the mice. Compared with VACV, VACV-PBCA-NP showed distinct characteristic of sustained-release in vivo and the drug entering hepatocytes were also greatly increased.
CONCLUSION: VACV-PBCA-NP has the characteristic of liver targeting and can increase the permeability of VACV to hepatocytes.
Keywords: valaciclovir, polybutylcyan-oacrylate nanoparticles, liver targeting, hepatocytes permeability
INTRODUCTION
Valaciclovir (VACV), the L-valyl ester of acyclovir (ACV), is a new antiviral drug which can be hydrolyzed into ACV and L-valyl rapidly in the presence of enzyme in vivo. Compared with ACV, VACV has the advantages of better solubility and higher bioavailability[1,2]. It has been demonstrated that ACV is effective for hepatitis B[3-6], but not so effective as for herpes virus infection. This is chiefly due to the poor penetration of ACV into the liver cells[7]. One approach solving this problem is to use drug carriers capable of enhancing the liver and liver intracellular drug delivery.
Nanoparticles (NP) is a new drug carrier[8,9]. We have investigated the action of VACV-loaded NP and demonstrated that the amount of drug increased 2.99 fold in the liver and decreased 5.46 fold in the kidney. Significant increase of intracellular drug was also observed.
MATERIALS AND METHODS
Materials
Both VACV obtained from Sichuan Institute for Antibiotics Industry and ACV supplied by Hubei Institute for Medicinal Industry, meet the USP reference standard. Butyl-cyanoacrylate (BCA) monomer was purchased from Shenzhen Nanguang Medicinal Colla Co. Ltd. and collagenase type I from Sigma Co. VACV-PBCA-NP injection was self-made.
Kunming mice, white Japanese rabbits and Wistar rats were all provided by Laboratory Animal Center, West China University of Medical Sciences.
Methods
Preparation of VACV-PBCA-NP VACV-PBCA-NP was prepared by emulsion polymerization method due to the good water solubility of VACV. An optimum procedure was developed based on the uniform design. Briefly, VACV, Dextran 70, pluronic F-68 and NaHSO3 were weighed and dissolved in water. The pH of the solution was adjusted to 2.2 by adding 0.1N-HCl, and then BCA was added slowly into the solution under electromagnetic stirring, the solution was stirred for additional 2 h at room temperature. Then the pH value of this colloidal solution was adjusted to 5-7 with 0.1N-NaOH. The solution was filtered through amicrofilter membrane (0.3 mm), filled into ampoules, freeze-dried and stored for later use.
Determination of embedding ratio and drug loading The embedding ratio and the drug loading of VACV were determined by HPLC at 254 nm. The HPLC conditions consisted of Shimpack CLC-ODS column (5 μm, 150 mm × 4.6 mm id), mobile phase CH3OH-0.02 mol·L-1 KH2PO4 (20:80), and flow rate 1 mL·min-1. The standard curve equation w as A = 2704.72 + 26406.30 C (r = 0.9999). The mean recovery was 97.41% ± 1.57%. The colloidal solution of VACV-PBCA-NP was freezingly ultracentrifuged, and the content of VACV in the supernatant was assayed. The embedding ratio (ER%) an d the drug loading (DL%) were calculated as follows:
ER% = [(VACV added - VACV in supernatant)/VACV added] × 100%
DL% = [(VACV added - VACV in supernatant)/BCA added] × 100%
Drug release from the VACV-PBCA-NP in vitro Dynamic dialysis bag technique was used to observe the drug release from VACV-PBCA-NP in vitro. The freeze dried powder of VACV-PBCA-NP was dispersed in physiological saline and the dispersion was transferred into a dialysis bag suspended in a conical container containing physiological saline solution. The container was shake n at 37 °C ± 1 °C. Samples were withdrawn at predetermined time, adjusted to pH9-11 with 0.1N-NaOH, boiled for 1 h and determined. The HPLC conditions were the same as mentioned above, the standard curve equation was A = 3046.73 + 62647.64 C (r = 0.9999). The accumulative drug release percentage was calculated to describe the drug release.
Measurement of drug in blood and viscera of mice Thirty Kunming mice were randomly divided into VACV-PBCA-NP group and VACV group, fifteen in each group. Each mouse was intravenously given VACV-PBCA-NP or VACV at a dose of 25 mg/kg body weight. The mice were killed and anatomized 15 min after the administration, and the heart, liver, lungs, kidneys and blood were taken out. Plasma of 0.5 mL or viscera homogenate were piped accurately and 1 mL chloroform and 0.5 mL-6% perchloric acid were added respectively. The mixture was vortexed and centrifuged, and 20 μLsupernatant was taken to determine VACV by HPLC.
Isolation and culture of hepatocytes The livers of Wistar rats (200 g ± 20 g in weight, fasted overnight) were taken out under aseptic condition, perfused with Hank′s solution until the blood washed out, cut into tiny pieces, digested with 0.1% collagenase at 37 °C for 45 min and filtered through a stainless steel mesh (mesh size: 100 μm). Hepatocytes were purified after being washed three times with Hank′s solution and one more time with RPMI-1640 solution. Cells were seeded at a density of 5 × 105 cells/each culture dish and incubated with 5% CO2 at 37 °C for 18 h. Then the medium was replaced with fresh RPMI-1640 solution, VACV- PBCA-NP or VACV was added at various concentrations for further incubation. Six, 12 and 24 h after the culture, the hepatocytes were taken out, washed three times with physiological saline and broken, then VACV in the cell was determined by HPLC.
Determination of blood concentration in rabbits Ten white Japanese rabbits were randomly devided into VACV-PBCA-NP group and VACV group, five in each group. Each rabbit was intravenously given VACV-PBCA-NP or VACV at a dose of 15 mg/kg body weight, 2 mL blood was taken at different time points after the injection and ACV in plasma was detected at 254 nm by HPL C method including Shimpack CLC-ODS analytical column (5 μm, 150 mm ± 4.6 mm id) and mobile phase methanol-water-acetic acid (1:99:0.5).
RESULTS
Morphology (Figure 1)
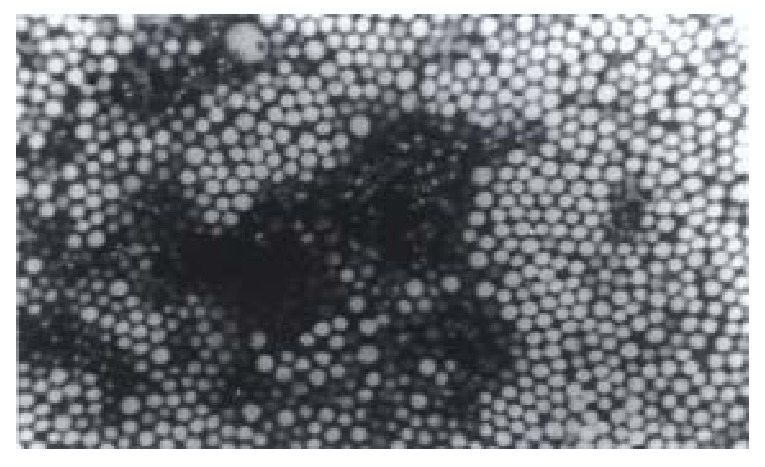
Figure 1.
Transmission electron micrographs of VACV- PBCA-NP.
The surface of the VACV-PBCA-NP is regular and non-adhesive. The average, the maximum and the minimum diameter of VACV-PBCA-NP is 104.77 nm, 141 nm and 76 nm respectively. Its diameter is not abnormally distributed.
Drug loading characteristics Table 1 shows the embedding ratio and drug loading of VACV-PBCA-NP.
Table 1.
Embedding ratio and drug loading of VACV-PBCA-NP
| Batch NO. | Embedding ratio (%) | Drug loading (%) |
| 961010 | 85.10 | 12.15 |
| 961012 | 83.75 | 10.51 |
| 961015 | 85.70 | 10.93 |
| Average | 84.85 | 11.20 |
Stability
Freeze-dried powder of VACV-PBCA-NP was stored at 3 °C-5 °C, 20 °C-25 °C and 37 °C (RH 75%) respectively for 3 months. There were no noticeable changes in the appearance, morphology, pH and VACV content under the condition of 3-5 °C and 20-25 °C, but changes were found at 37 °C (Table 2).
Table 2.
Stability results of VACV-PBCA-NP
| Temperature (°C) |
0 month |
3 months |
||||||
| D/nm | C | pH | Color | D/nm | C | pH | Color | |
| 3-5 | 107.28 | 0.49 | 5.34 | white | 110.42 | 0.47 | 5.25 | white |
| 15-25 | 107.28 | 0.49 | 5.34 | white | 109.37 | 0.48 | 5.05 | white |
| 37 (RH75%) | 107.28 | 0.49 | 5.34 | white | 105.61 | 0.43 | 4.10 | white |
C: Mg/ampoule; D: Diameter.
Drug release characteristics
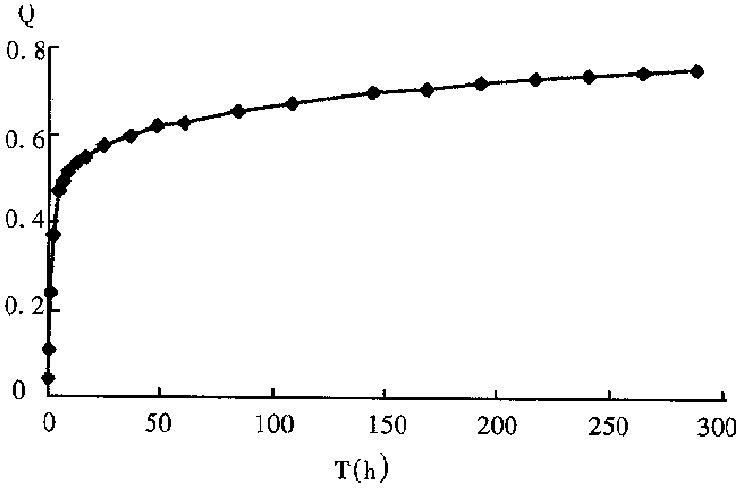
Figure 2 shows the drug release profile of VACV-PBCA-NP freeze-dried injection. The curve (Figure 2) corresponded to the two-phase kinetics equation: 1 - Q = 0.3663e-0.0015t + 0.3000e-0.0524t.
Figure 2.
Release profile of VACV-PBCA-NP freeze-dried injection. Q: Percentage of accumulative drug release.
Distribution of VACV-PBCA-NP in viscera and blood of mice
The amount of ACV in each organ recorded as ACVi and ACVt was obtained by adding ACVi in all viscera at different time points. The ratio of ACVi/ACVt × 100% represented the relative content of VACV-PBCA-NP in viscera and blood (Table 3). Table 3 shows that the relative content of VACV-PBCA-NP in liver was 74.49%, 2.99 times higher than that of VACV, and in kidney was 9.36%, 5.46 times lower than that of VACV.
Table 3.
Relative content in various organs 15 min after iv administration of VACV-PBCA-NP and VACV respectively (%, n = 3)
| Sample | Heart | Liver | Spleen | Lung | Kidney | Blood |
| VACV-PBCA-NP | 0.99 | 74.49 | 2.46 | 2.89 | 9.36 | 9.82 |
| VACV | 2.14 | 24.92 | 2.44 | 3.01 | 51.15 | 16.54 |
The permeability of VACV-PBCA-NP to hepatocytes
Because of the same amount of the cells added to each culture dish (5 × 105), the peak area of ACV was used to represent the effect of the VACV taken in by rat liver cell, the results showed that the drug amount of VACV-PBCA-NP in rat hepatocytes group was 28.77, 21.90 and 5.22 times that of VACV control group a t 6 h, 12 h and 24 h, respectively.
The pharmacokinetic parameters of VACV-PBCA-NP and VACV in rabbit after iv administration
The concentration-time data of the two groups both fitted the two-compartment model, the equation was C = 20.88e-7.653t + 0.63e-0.0001t and C = 10.19e-2.67226t + 0.88e-0.3789t. The main pharmacokinetic parameters were analyzed by the single factor variance method. The results are shown in Table 4.
Table 4.
The results of single factor variance analysis of the main pharmacokinetic parameters of VACV-PBCA-NP and VACV
| Factors | A | B | DF | F (test) | F (criterion) | P |
| AUC | 223.34 | 6.40 | 1.8 | 35.87 | 11.30 | < 0.01 |
| MRT (h) | 244.58 | 0.95 | 1.8 | 28.83 | 11.30 | < 0.01 |
A: VACV-PBCA-NP; B: VACV; DF: Degree of freedom.
Table 4 shows that there is a significant difference in the main pharmacokinetic parameters between the two groups.
DISCUSSION
The VACV-PBCA-NP freeze-dried injection stored at 37 °C/RH75% would change in the appearance, pH and the drug content. The results implied that temperature an d humidity affect the stability of VACV-PBCA-NP. The reason may be that higher temperature and humidity would speed up the generation of L-valyl and ACV. Therefore, VACV-PBCA-NP should be preserved at low temperature and humidity.
VACV will degrade into ACV at 37 °C. The experiment showed that VACV would be completely turned into ACV when heated for 1 h at 100 °C, but ACV was stable in this situation. Therefore, in the in vitro experiment of the drug release from nanoparticles, the samples were treated by the method mentioned above. The released amount of VACV could be calculated by measuring ACV.
VACV will turn into ACV rapidly and completely in vivo because of the presence of enzyme. Therefore, we determined ACV in blood and viscera of animal after i.v. VACV and VACV-PBCA-NP by a HPLC method of good recovery.
Footnotes
Dr. Zhi-Rong Zhang, male, graduated from West China University of Medic al Sciences (WCUMS) as a PhD in 1993, now professor of pharmaceutics, Dean of the School of Pharmacy, WCUMS. Member of Chinese Pharmacopoeia Commission, Council Member of Chinese Pharmaceutical Association (CPA), Member of Society of Pharmaceutics of CPA, specialized in targeted delivery system and has more than 80 papers and 6 books published.
Supported by the National Natural Science Foundation of China, NO. 39470831
Edited by MA Jing-Yun
References
- 1.Beauchamp LM, Orr GF, Mirunda P. Amino and ester prodrugs of acyclovir. Antiviral Chem Chemother. 1992;3:157–164. [Google Scholar]
- 2.Weller S, Blum MR, Doucette M, Burnette T, Cederberg DM, de Miranda P, Smiley ML. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther. 1993;54:595–605. doi: 10.1038/clpt.1993.196. [DOI] [PubMed] [Google Scholar]
- 3.Yang SS, Guan MY, Yang LH. Comparison of acyclovir and interferon in the treatment of chronic hepatitis B. Hubei Yixueyuan Zazhi. 1987;8:144–147. [Google Scholar]
- 4.Zhou JL, Xu LZ, Wu H, Min J. The effect of acyclovir to chronic active hepatitis B. Tianjin Yiyao. 1989;17:687–689. [Google Scholar]
- 5.Weller IV, Carreno V, Fowler MJ, Monjardino J, Makinen D, Varghese Z, Sweny P, Thomas HC, Sherlock S. Acyclovir in hepatitis B antigen-positive chronic liver disease: inhibition of viral replication and transient renal impairment with iv bolus administration. J Antimicrob Chemother. 1983;11:223–231. doi: 10.1093/jac/11.3.223. [DOI] [PubMed] [Google Scholar]
- 6.Smith CI, Scullard GH, Gregory PB, Robinson WS, Merigan TC. Preliminary studies of acyclovir in chronic hepatitis B. Am J Med. 1982;73:267–270. doi: 10.1016/0002-9343(82)90103-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZR, Liao GT, Hou SX. [Study on mitoxantrone polycyanoacrylate nanospheres] Yao Xue Xue Bao. 1994;29:544–549. [PubMed] [Google Scholar]
- 8.Marty JJ, Oppenheim RC, Speiser P. Nanoparticles--a new colloidal drug delivery system. Pharm Acta Helv. 1978;53:17–23. [PubMed] [Google Scholar]
- 9.Kreuter J. Nanoparticles and nanocapsules--new dosage forms in the nanometer size range. Pharm Acta Helv. 1978;53:33–39. [PubMed] [Google Scholar]


