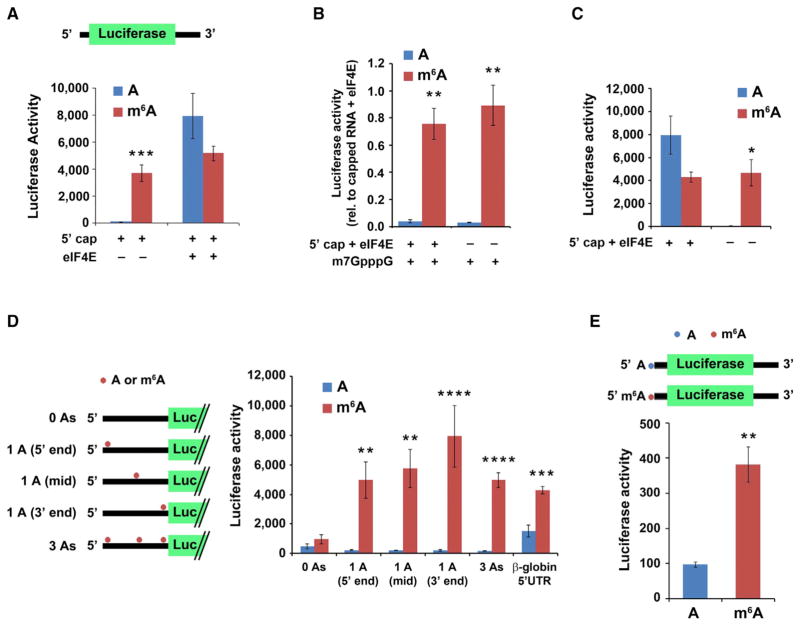
Figure 2. m6A within the 5′ UTR Enables Cap-Independent Translation of mRNA.
(A) 5′ UTR m6A permits mRNA translation without the need for the cap-binding protein eIF4E. In vitro translation was performed using a HeLa cell extract mixed with luciferase-encoding, capped mRNA containing either A or m6A. Protein production was measured by quantifying luciferase activity. Cap-dependent translation is observed from both methylated and unmethylated mRNAs in the presence of eIF4E. However, when eIF4E is absent, only the m6A-containing mRNA is translated (n = 4; mean ± SD; ***p < 0.0001).
(B) Presence of a 5′ cap analog is unable to abolish m6A-induced mRNA translation. Luciferase mRNAs were translated as in (A). 1 mM free cap analog (m7GpppG) was added to sequester cap-binding proteins. Addition of m7GpppG abolishes cap-dependent translation of unmethylated mRNA (left) but is unable to abolish the cap-independent translation induced by m6A (right). Levels of luciferase activity are shown relative to capped mRNA + 10 pmole eIF4E (n = 3; mean ± SD; *p < 0.01, **p < 0.001).
(C) In vitro translation was performed using luciferase-encoding mRNA containing A or 50% m6A and with or without a 5′cap as indicated. While unmethylated, capped mRNA + 10 pmole eIF4E is robustly translated, the unmethylated, uncapped mRNA fails to be translated. However, m6A-containing mRNA is efficiently translated even when no 5′ cap is present (n = 3; mean ± SD; *p < 0.01).
(D) m6A residues in the coding sequence do not induce cap-independent translation. Uncapped, luciferase-encoding mRNAs containing either the natural β-globin 5′ UTR or a modified β-globin 5′ UTR containing either zero, one, or three A residues as indicated were used for in vitro translation assays. Translation of m6A-containing mRNA with zero A residues in the 5′ UTR was markedly diminished, indicating that coding sequence m6A residues are unable to induce cap-independent translation. However, when a single m6A was added to the 5′ UTR, the transcripts were robustly translated. Methylated 5′ UTRs with a single A near the 5′ end, the middle (mid), or near the 3′ end all showed similar levels of translation (n = 3; mean ± SD; **p < 0.001, ***p < 0.0001, ****p < 0.00001). Schematic shows the distribution of A residues within each β-globin 5′ UTR variant (the unmodified β-globin 5′ UTR contains 17 A residues).
(E) mRNA with a single m6A within the 5′ UTR and no m6As in the remainder of the transcript induces cap-independent translation. Uncapped, luciferase-encoding mRNAs, which contained either a single adenosine 5′-monophosphate (AMP) or N6-methyladenosine 5′-monophosphate (m6AMP) at the 5′ end, were used for in vitro translation. Only the m6A-containing mRNA was translated, demonstrating that a single 5′ end m6A residue is capable of inducing cap-independent translation (n = 3; mean ± SD; **p < 0.001). The reduced translation efficiency of this mRNA compared to mRNAs with internally methylated 5′ UTRs is likely due to inefficient incorporation of m6A residues at the 5′ end by T7 RNA polymerase.
See also Figure S1.