Figure 3. m6A-Mediated Translation Occurs through a 5′ End-Dependent Mechanism.
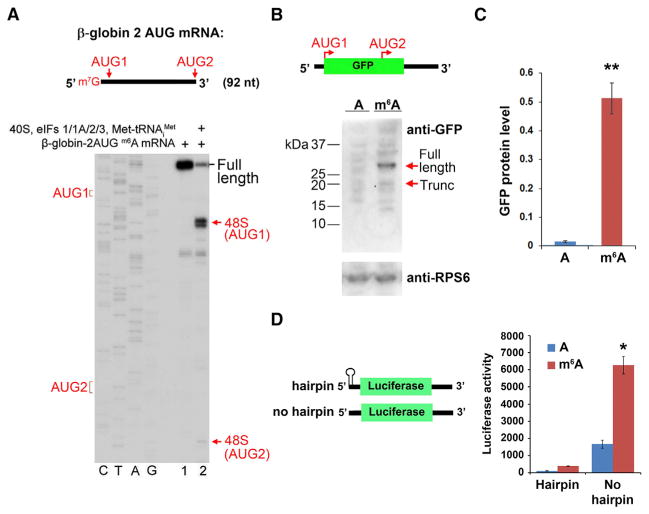
(A) Toeprinting assays were performed using a capped, m6A-containing mRNA containing the β-globin 5′ UTR sequence, which was modified to include two AUG initiation codons (“AUG1” and “AUG2” in the schematic). The majority of 48S complexes were assembled at AUG1, with negligible levels of 48S complexes detected at AUG2.
(B) Uncapped, A-, or m6A-containing mRNAs encoding GFP were used for in vitro translation. The mRNA contains two near-kozak start codons: AUG 1 encodes the full-length GFP protein, and internally localized AUG2 encodes an in-frame truncated (~17 kDa) protein comprising the C-terminal portion of GFP. Full-length and truncated GFP protein levels (sizes indicated by arrows) were measured by western blot. m6A primarily promotes translation of the full-length protein and fails to induce internal entry-mediated translation from AUG2. Levels of the ribosomal protein RPS6 are shown as a loading control.
(C) Quantification of full-length GFP protein levels in (B) shows increased protein expression of methylated mRNA versus unmethylated mRNA (n = 3; mean ± SD; **p < 0.001).
(D) The presence of a stable hairpin at the beginning of the 5′ UTR to block 5′ end entry severely attenuates m6A-mediated translation (n = 3; mean ± SD; *p < 0.01).
See also Figure S1.