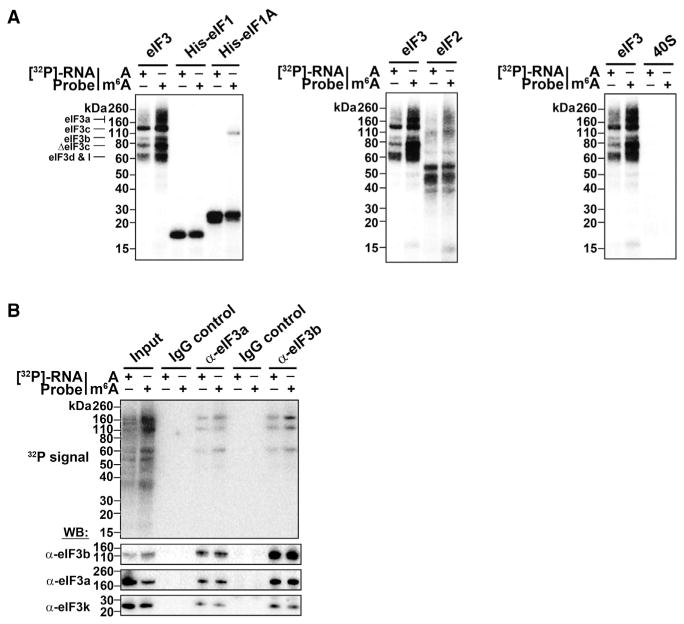
Figure 4. The 43S Complex Component eIF3 Binds m6A.
(A) Indicated proteins/protein complexes were incubated with radiolabeled A- or m6A-containing RNA probes and crosslinked. Unbound RNAs were then removed with RNase I, proteins were separated by SDS-PAGE, and radioactively-labeled RNAs were detected. eIF1, eIF1A, eIF2, and the 40S ribosomal subunit show no preferential crosslinking to methylated RNA. However, eIF3 preparations exhibit strong crosslinking to methylated RNA at bands around 60 kD, 80 kD, and 110–160 kD, which correspond to multiple subunits of the eIF3 complex as indicated.
(B) Crosslinking assays were performed as in (A) using the HeLa cell extracts utilized in in vitro translation assays. The eIF3 complex was immunoprecipitated using antibodies against eIF3a or eIF3b, and proteins containing crosslinked RNA were detected. Both eIF3 antibodies precipitated proteins that preferentially crosslinked to m6A RNA. Immunoprecipitation using rabbit and mouse IgG control antibodies are shown as negative controls. Western blotting for the indicated proteins indicates their enrichment following immunoprecipitation (bottom). The input lanes throughout have 25% of the material loaded for the IP lanes.
See also Figures S2 and S3.