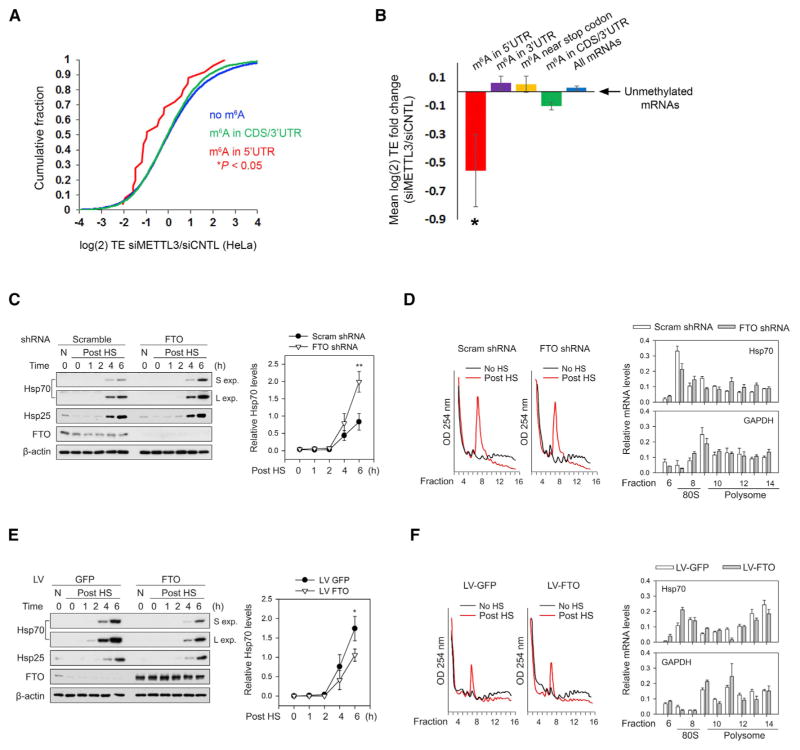
Figure 6. m6A Mediates Stress-Induced Translation of Hsp70.
(A) Depletion of the m6A methyltransferase, METTL3, decreases the TE of mRNAs with 5′ UTR m6A. Ribosome profiling data from HeLa cells expressing METTL3 or control siRNAs (Wang et al., 2015) were used to determine changes in TE for various classes of mRNAs defined by single-nucleotide-resolution m6A mapping. Compared to nonmethylated mRNAs (blue), transcripts with m6A residues in the coding sequence (CDS) or 3′ UTR (green) exhibit only a marginal decrease in TE. However, mRNAs containing m6A within the 5′ UTR (red) show a large reduction in TE. p values were calculated using the Mann-Whitney test.
(B) TEs of various classes of m6A-containing mRNAs were analyzed using ribosome profiling datasets from HeLa cells as described in (A). Shown are the mean fold changes in TE (siMETTL3/siControl) for mRNAs with m6A residues only in the 5′ UTR (red), within the 3′ UTR (purple), within 50 nt of the stop codon (yellow), within the CDS and/or 3′ UTR (green), or in all mRNAs (blue), as defined by single-nucleotide-resolution m6A mapping. mRNAs with 5′ UTR m6A residues exhibit a dramatic reduction in TE after METTL3 depletion, whereas transcripts with m6As in other regions fail to show this effect. All mean fold change TE values were computed after background subtraction of the mean fold change computed from all nonmethylated control mRNAs, as indicated by the arrow (mean ± SEM; *p < 0.05).
(C) Fto knockdown increases heat-shock-induced translation of Hsp70. MEF cells stably expressing either Fto shRNA or scramble shRNA were subjected to heat shock stress. Cell lysates were collected at various times post-heat shock (“Post HS”) and then used for western blot analysis with the indicated antibodies. Fto knockdown increased the levels of stress-induced Hsp70 protein compared to control shRNA (“S exp” = short exposure; “L exp” = long exposure). Levels of Hsp25, another heat shock-induced protein, were unaffected by Fto knockdown. Right panel shows quantification of Hsp70 levels normalized to β-actin (n = 3; mean ± SEM; **p < 0.1).
(D) MEFs stably expressing control or Fto shRNA were subjected to heat shock stress as in (C). Polysome fractions were separated using sucrose gradient fractionation (left panels) followed by RT-qPCR for Hsp70 (top right panel) and Gapdh (bottom right panel) in each fraction. Hsp70 levels are increased in polysome fractions following Fto knockdown, whereas the distribution of Gapdh is unchanged (n = 3; mean ± SEM; Hsp70: p = 0.0007, two-way ANOVA; Gapdh: p = 0.3722, two-way ANOVA considering the entire range of time points).
(E) MEF cells were infected with either GFP or Fto lentivirus and subjected to heat shock stress. Cell lysates were collected at various times post-heat shock and then used for western blot analysis with the indicated antibodies. Fto overexpression decreased the levels of heat-shock-induced Hsp70 protein compared to GFP overexpression. Levels of Hsp25 were unaffected by Fto overexpression. Right panel shows quantification of Hsp70 levels normalized to β-actin (n = 3; Mean ± SEM; *p < 0.5).
(F) MEFs with or without Fto overexpression were subjected to heat shock stress as in (E). Polysome fractions were separated using sucrose gradient fractionation (left panels) followed by RT-qPCR of Hsp70 (top right panel) and Gapdh (bottom right panel) in each fraction. Hsp70 levels are decreased in polysome fractions following Fto overexpression, whereas the distribution of Gapdh is unchanged (n = 3; mean ± SEM; Hsp70: p < 0.0001, two-way ANOVA; Gapdh: p = 0.1910, two-way ANOVA considering the entire range of time points).
See also Figures S5 and S6 and Table S1.