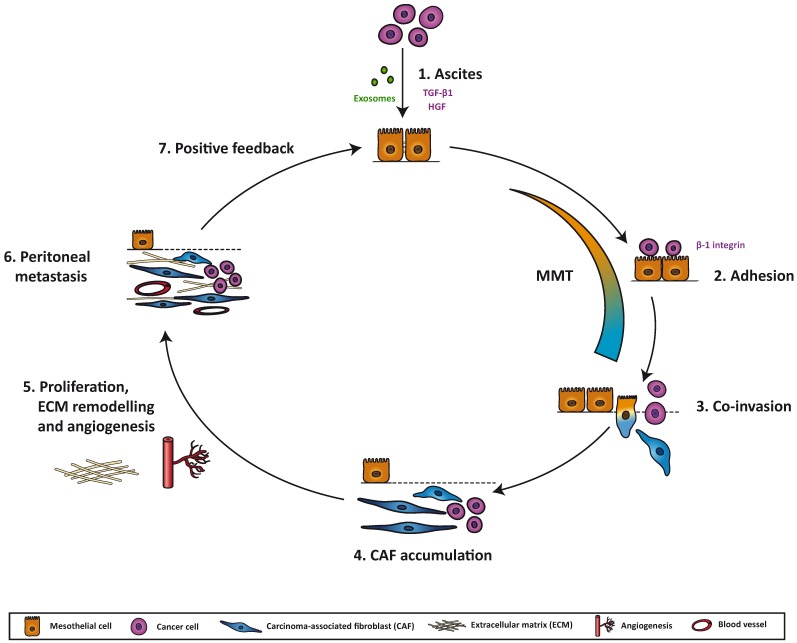
Figure 1.
Model for transformation of the pre-metastatic niche by mesothelial-to-mesenchymal transition in the peritoneum: (1) Cancer cells at the primary site secrete an array of exosomes, cytokines, chemokines and growth factors that accumulate in the ascitic fluid. Some of these molecules, such as transforming growth factor-beta1 (TGF-β1) or hepatocyte growth factor (HGF), induce changes in the mesothelial cells (MCs), triggering a mesothelial-to-mesenchymal transition (MMT), through which they lose apico-basal polarity and cell-cell adhesion, and acquire myofibroblastic properties. (2) Cancer cells adhere via β1 integrins to the MCs that line the peritoneum. This adhesion is increased when a MMT has taken place. (3) In later stages of MMT, MCs have converted into carcinoma-associated fibroblasts (CAFs) that represent the invasion front into the stroma, followed by cancer cells. (4) After invading, MC-derived CAFs accumulate in the peritoneal stroma. (5) CAFs produce factors that affect peritoneal implant progression: they induce angiogenesis via secretion of VEGF, among other factors; they transform the extracellular matrix (ECM) by producing collagen, fibronectin and other structural proteins, and they remodel the ECM via matrix metalloproteinases (MMPs); they also stimulate proliferation of cancer cells. (6) The metastatic niche in the peritoneum is created as a result of the clearance of the MC monolayer, myofibroblast conversion of MCs, invasion of MCs and cancer cells, accumulation of CAFs, increased vascularization, ECM remodeling and proliferation of cancer cells. (7) Cancer cells in the new site, together with CAFs, will continue to produce factors that modify the stroma and induce changes in both cancer cells and MCs, thereby creating a positive feedback loop.