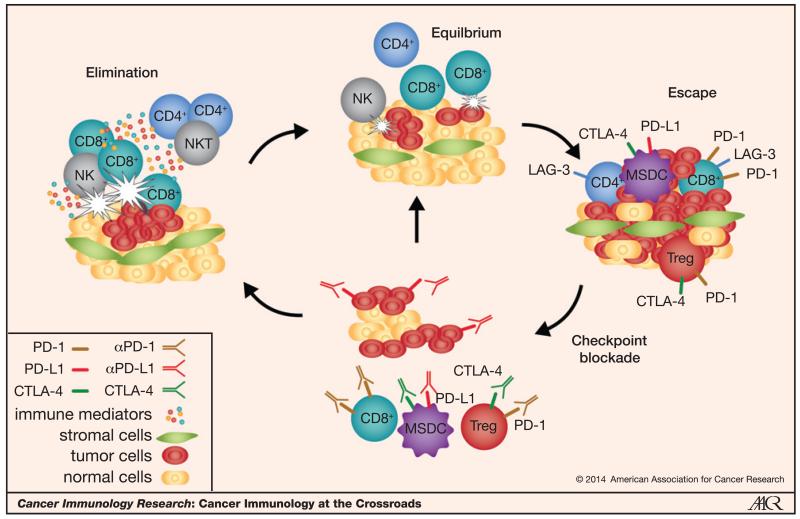
Figure 2.
The concept of immunoediting at work and the potential effects of immune checkpoint blockade on the equilibrium between the tumor and the host immune system. The relationship between a tumor and the host immune system may be best conceptualized by the immunoediting hypothesis, which encompasses three phases: tumor elimination, equilibrium, and tumor escape. In the elimination stage, immune cells such NK cells or CD8+ effector cells recognize and eliminate small or highly immunogenic tumors before they are even detectable radiographically. However, some tumors elude the initial host defense and transition to a state of equilibrium and coexist with the immune system; tumors try to grow but are generally functionally restrained by the immune system. Tumor-cell variants can evolve that can resist immune cell recognition by highjacking host mechanisms including upregulation of components of immunoinhibitory pathways such as PD-L1–PD-1, class II MHC/LAG-3, Galectin-9/Tim-3, and VEGF. Having escaped the immune system’s defense mechanisms, these tumors proliferate and are the ones we face in the clinic. Novel blocking antibodies targeting immune checkpoints, such as PD-1 and CTLA-4, are being evaluated in RCC and have shown encouraging preliminary efficacy. These therapies increase the host antitumor T-cell response and may induce disease stabilization (“equilibrium”) and in some cases, force the balance back to the tumor elimination stage, which may be reflected in the objective responses including CRs that have been observed in patients.