Abstract
Background
The widespread use of diagnostic imaging has led to an increase in the incidence and diagnosis of benign liver tumors. The objective of this study was to define the overall use and temporal trends of operative procedures for benign liver tumors using a nationally representative cohort.
Methods
All patients who underwent liver surgery for benign liver tumors between 2000 and 2011 were identified from the Nationwide Inpatient Sample database. Trends in annual volume of liver procedures were analyzed using the average annual percent change (AAPC) assessed by joinpoint analysis.
Results
There were 2,489 open (94.5%) and 144 (5.5%) minimally invasive surgical (MIS) procedures. Partial hepatectomy accounted for 43.8% of all cases (n = 1,153). Surgery for patients with benign liver tumors increased from 156 in 2000 to 272 in 2011 (AAPC, 5.8%; 95% CI, 3.2–8.6%). There was decline in the relative use of open operative procedures from 98.1% in 2000 to 92.3% in 2011 (AAPC, −0.4%; 95% CI, −0.7 to −0.1%). In contrast, the proportion of MIS procedures increased from 1.9% in 2000 to 7.7% in 2011 (AAPC, 7.4%; 95% CI, 1.9–13.3%). The median duration of stay among all patients was 5 days (interquartile range, 4–7; 5 days [open] vs 3 days [MIS]; P <.001). Inpatient mortality was 0.6% (n = 15 [open] vs n = 0 [MIS]; P = .43) and did not change during the study period (P > .05).
Conclusion
Overall volume of surgical management of benign liver tumors has increased substantially over the past decade. There has been a relative shift away from open procedures toward MIS procedures.
Benign liver tumors are relatively common lesions that can be observed in ≤20–50% of the population at the time of autopsy.1–4 Benign liver tumors are classified generally into cystic or solid tumors based on their radiologic features. Cystic tumors tend to be more frequent and can occur in ≤5 to 15% of the population.5,6 Although benign solid lesions probably occur with less overall frequency, these lesions can be often found in women of childbearing age.7 Over the past decade, the incidence of benign liver tumors has increased,8,9 probably related in part to the widespread use of abdominal imaging such as ultrasonography, CT, and MRI.10–13 With the discovery of more and more benign liver lesions, the clinician is faced increasingly with the need to make therapeutic decisions regarding the management of these lesions. Although advanced imaging techniques and an improved understanding of the natural history of many benign liver tumors has facilitated therapeutic decision making, the management of these lesions can still be challenging.14–17
The current indications for an operative approach to benign liver tumors include progressive symptoms and suspicion of malignant change.18,19 In the setting of benign disease, the overall indication and utilization of surgery may be somewhat subjective and variable. Benign liver tumors are often discovered incidentally on cross-sectional imaging obtained for other reasons. Many patients may also present with nonspecific pain in the setting of a benign liver lesion. Management of patients with benign liver lesions can therefore be challenging and controversial, because the indications for operative intervention may be more ambiguous (eg, pain, diagnostic uncertainty, patient anxiety).20 In this setting, the utilization of surgery for benign liver tumors may vary. Furthermore, the expanding utilization of cross-sectional imaging and subsequent increased discovery of benign liver lesions, as well as the increased use of minimally invasive surgical (MIS) approaches, may impact the relative use of surgery for benign liver lesions. Previous studies on surgery of benign liver lesions have not investigated temporal trends in the utilization of surgery for benign liver lesions. Rather, past data have focused almost exclusively on the morbidity and mortality associated with surgery for benign liver lesions.21,22 In addition, most series that assessed the use of operative procedures for benign liver tumors were conducted in a single center over a short period of time.23–25 Given this, we sought to characterize the use of operative procedures for benign liver tumors over the last decade using a representative, population-based dataset. Specifically, the objective of the current study was to define the overall and temporal trends in the utilization of surgery, as well as examine in-hospital outcomes, for benign liver tumors using data from the National Inpatient Sample (NIS) database.
MATERIALS AND METHODS
Data sources and samples
We conducted a retrospective analysis using the NIS database to examine the utilization of surgery for benign liver tumors between January 2001 and December 2011. The NIS database is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP) that contains deidentified data on patients enrolled in Medicare, Medicaid, private insurances, and the uninsured. NIS is the largest publicly available all-payer inpatient care database in the United States.26 The NIS contains comprehensive data on 8 million hospital stays gathered from 1,000 hospitals, which represents approximately 20% of stratified sample of all US community hospitals. Data in NIS is obtained from states that participate in HCUP, which represent over 97 percent of the US population. The NIS collects >100 variables, ranging from patient demographics, diagnosis codes, procedure type, and hospital features.
All patients who were discharged with an International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic code for liver benign tumors and biliary passage (2,115) and congenital cystic liver disease (75,162) were included in our study. Patients with ICD-9-CM diagnosis codes for hemangioma (22,804) and lymphangioma (2,281) were included only if patients had procedure codes for liver resection. Liver operative procedure codes were identified using ICD-CM procedure code: partial hepatectomy (50.22), hepatic lobectomy (50.3), hepatic wedge resection (5,012), liver ablation (50.23–50.26), and ‘‘other’’ liver procedures such as enucleation (50.29). MIS was defined as a composite of laparoscopic and robotic procedures using ICD-9-CM coding (laparoscopy, 54.21; robotic, 17.4, 17.41, 17.42, 17.43, and 17.49), as well as laparoscopic ablation of liver lesions (5,025).27,28 Information regarding laparoscopic procedures was available for all time periods; however, data for robotic information was only available from October 2008 onward. For all patients, demographic-specific data on age, sex, race, primary payer, hospital teaching status, admission type, and hospital location were abstracted when available. Patient comorbidities (hypertension, diabetes, chronic lung disease, obesity, chronic kidney disease, and congestive heart failure) were defined according to ICD-9-CM codes. Mortality (in-hospital mortality) and duration of stay were extracted directly from the database. Trends in mortality, duration of stay, and procedures type were determined for 3 time intervals (2000–2003, 2004–2007, and 2008–2011). In-hospital perioperative complications were defined using the corresponding ICD-9-CM diagnostic codes and included infection, wound complication, bleeding complication, myocardial infarction, postoperative respiratory complications, cerebrovascular accident, venous thromboembolism, acute renal failure, urinary tract infection, liver failure, digestive complication, postoperative shock, and reoperation.
Statistical analysis
Characteristics of patients, hospitals, and in-hospital outcomes were summarized using standard measures of frequencies and percentage or median and interquartile ranges (IQR), as appropriate. Chi-square analysis was used to assess temporal trends in characteristics of patients, hospitals, and inpatient outcome within a given time period. We utilized joinpoint trends analysis to calculate annual percentage change (APC) statistics that characterize the magnitude and direction of trends in annual volumes and relative utility of operative procedures between 2000 and 2011.29 Through joinpoint analysis, we calculated APC and average APC (AAPC) between 2000 and 2011. For this study, a maximum of 1 joinpoints (2 line segments) were allowed for each analysis. Joinpoint Regression Program was used for the joinpoint analysis (Version 4.0.4, May 2013; Surveillance Research Program, National Cancer Institute, Bethesda, MD), and other statistical analyses employed STATA version 12.0 (StataCorp, College Station, TX).
RESULTS
Patient and hospital characteristics from 2000 to 2011
During the 12-year period, a total of 2,633 hepatic procedures were performed for benign liver tumors. The characteristics of the patients and hospitals are shown in Table I. Median age of the study population was 45 years (IQR, 35–56) and 82.3% (n = 2,157) of patients were female. The majority of patients were Caucasian (n = 1,542; 73.7%). Although patient age and race were similar across time periods in the study cohort, there was an increase in the proportion of female patients (P = .04). Most cases were performed as an elective operation (n = 2,010; 85.7%) in an urban hospital (n = 1,656; 97.2%). Liver resection was conducted the most in the South region, with more cases being done in the South region over time (P < .01). The majority of hepatic procedures were performed at a teaching hospital (n = 1,519; 89.1%), with a slight increase over time (2004–2007, 87.1% vs 2008–2011, 90.4%: P = .04). The most common comorbidities were hypertension (n = 663, 25.2%), followed by diabetes (n = 217, 8.2%) and obesity (n = 155, 5.9%). Of note, obesity and chronic kidney disease increased during the study period (P < .05). Overall incidence of any complications during the hospitalization was 29.9% (30.5% [open] vs 20.1% [MIS]; P = .008) and did not change over the time periods examined (P = .11). The median duration of stay among all patients was 5 days (IQR, 4–7; 5 days [open] vs 3 days [MIS]; P < .001). Inpatient mortality was 0.6% (n = 15 [open] vs n = 0 [MIS]; P = .43) and did not change during the study period (P > .05).
Table I.
Characteristics of patients who had hepatic procedures for benign liver tumors in the United States
| Characteristic | Overall (n = 2,633) | Period 1 (2000–2003; n = 705) | Period 2 (2004–2007; n = 858) | Period 3 (2008–2011; n = 1,070) | P value |
|---|---|---|---|---|---|
| Gender | .04 | ||||
| Female | 2,157 (82.3) | 557 (79.1) | 709 (83.2) | 891 (83.6) | |
| Age (y), median (IQR) | 45 (35–56) | 45 (35–54) | 46 (36–56) | 45 (34–56) | .55 |
| Race | .81 | ||||
| Caucasian | 1,542 (73.7) | 278 (73.7) | 473 (73.2) | 691 (74.1) | |
| African American | 226 (10.8) | 53 (10.3) | 65 (10.1) | 108 (11.6) | |
| Hispanic | 180 (8.6) | 44 (8.6) | 63 (9.8) | 73 (7.8) | |
| Other | 144 (6.9) | 38 (7.4) | 45 (7.0) | 61 (6.5) | |
| Comorbidities | |||||
| Hypertension | 663 (25.2) | 167 (23.7) | 210 (24.5) | 286 (26.7) | .30 |
| Diabetes | 217 (8.2) | 53 (7.5) | 64 (7.5) | 100 (9.4) | .23 |
| Chronic lung disease | 58 (2.2) | 22 (3.1) | 17 (2.0) | 19 (1.8) | .15 |
| Obesity | 155 (5.9) | 25 (3.6) | 45 (5.2) | 85 (7.9) | <.001 |
| Admission type (n = 2,345) | .17 | ||||
| Elective | 2,010 (85.7) | 358 (84.8) | 720 (84.3) | 932 (87.2) | |
| Nonelective | 335 (14.3) | 64 (15.2) | 134 (15.7) | 137 (12.8) | |
| Hospital location (n = 1,704) | .28 | ||||
| Urban | 1,656 (97.2) | NA | 631 (96.6) | 1,925 (97.5) | |
| Rural | 48 (2.8) | NA | 22 (3.4) | 26 (2.5) | |
| Geographic region ( n = 1,723) | <.001 | ||||
| Northeast | 364 (21.1) | NA | 114 (17.5) | 250 (23.4) | |
| Midwest | 432 (25.1) | NA | 144 (22.0) | 288 (26.9) | |
| South | 493 (28.6) | NA | 190 (29.1) | 303 (28.3) | |
| West | 434 (25.2) | NA | 205 (31.4) | 229 (21.4) | |
| Hospital teaching status, (n = 1,704) | .04 | ||||
| Teaching | 1,519 (89.1) | NA | 569 (87.1) | 950 (90.4) | |
| Nonteaching | 185 (10.9) | NA | 84 (12.9) | 101 (9.6) | |
| Any complication | 788 (29.9) | 200 (28.4) | 235 (27.4) | 353 (33.0) | .11 |
| Duration of stay, median (IQR) | 5 (4–7) | 5 (4–7) | 5 (4–7) | 5 (3–7) | .36 |
| Inpatient mortality | 15 (0.6) | 6 (0.9) | 3 (0.4) | 6 (0.6) | .42 |
IQR, Interquartile range; NA, not applicable.
Operative approach
At the time of the operation, the vast majority of cases were performed as an open procedure (n = 2,489; 94.5%); the remaining 144 cases (5.5%) were MIS (Table II). Patients who underwent an MIS procedure were often older (45 years [open] vs 50 years [MIS]; P = .004) and more likely to have Medicare coverage (14.4% [open] vs 22.6% [MIS]; P = .02). Other patient and hospital characteristics were comparable in the open versus MIS groups. The most common hepatic operation was a partial hepatectomy, accounting for 43.8% of all cases (n = 1,153) followed by hepatic lobectomy (n = 660; 25.1%). Hepatic wedge resection and liver ablation accounted for 7.9% (n = 207) and 1.9% (n = 50) of cases, respectively. Among open procedures, partial hepatectomy was the most common hepatic procedure (n = 1,110; 44.6%) with hepatic lobectomy accounting for one-fourth of all open procedures (n = 648; 26.0). Hepatic wedge resection (n = 188; 7.6%) and liver ablation (n = 15; 0.6%) were conducted less often among open cases. Among all MIS cases, partial hepatectomy was most commonly performed (n = 43, 29.9%), whereas approximately one-third of MIS cases involved liver ablation (n = 40, 27.8%).
Table II.
Volume and proportion of inpatient hepatic procedures for benign liver tumors, 2000–2011
| Overall | Period 1 (2000–2003) | Period 2 (2004–2007) | Period 3 (2008–2011) | |
|---|---|---|---|---|
| Total (n) | 2,633 | 705 | 858 | 1,070 |
| Hepatic wedge resection | 207 (7.9) | 79 (11.2) | 69 (8.0) | 59 (5.5) |
| Partial hepatectomy | 1,153 (43.8) | 280 (39.7) | 339 (39.5) | 534 (49.9) |
| Hepatic lobectomy | 660 (25.1) | 183 (26.0) | 235 (27.4) | 242 (22.6) |
| Other procedure* | 613 (23.3) | 163 (23.1) | 215 (25.0) | 235 (21.9) |
| Open, n (%) | 2,489 (94.5) | 674 (95.6) | 817 (95.2) | 998 (93.2) |
| MIS, n (%) | 144 (5.5) | 31 (4.3) | 41 (4.8) | 72 (6.8) |
Other procedure includes liver ablation, cauterization of hepatic lesion and enucleation of hepatic lesion.
MIS, Minimally invasive surgery.
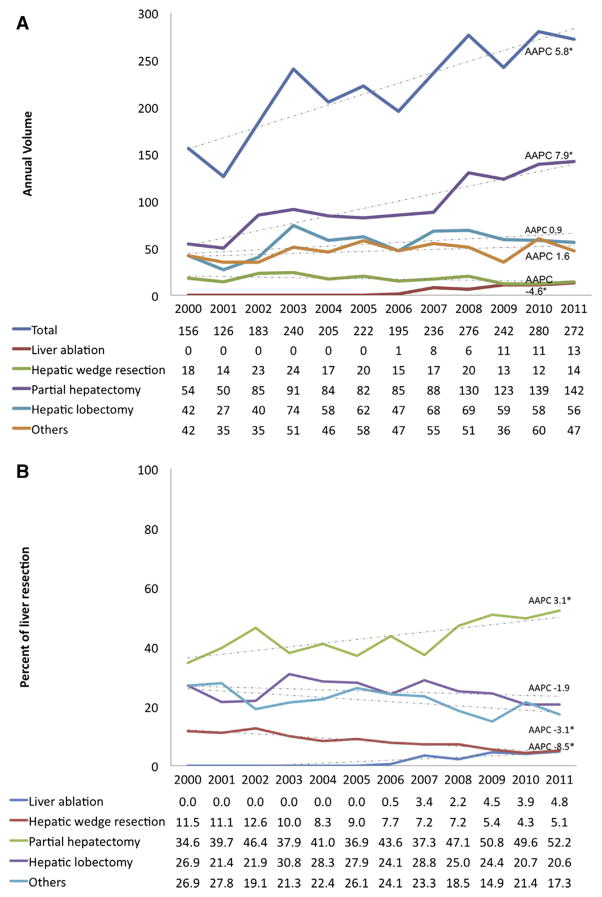
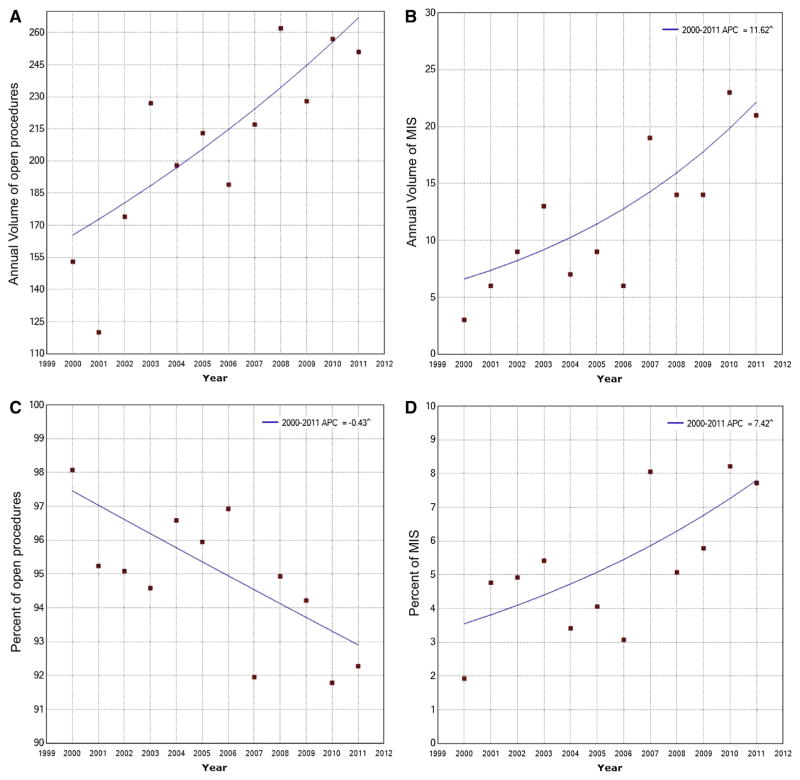
Trends in individual operative procedures as well as the proportion of each procedure were then evaluated to assess general shifts in utilization of surgery for benign liver tumors (Fig 1). Of note, the number of hepatic procedures performed for patients with benign liver tumors increased from 156 in 2000 to 272 in 2011 (AAPC, 5.8%; 95% CI, 3.2–8.6%). Although the number of open procedures for benign liver tumors increased from 153 in 2000 to 251 in 2011 (AAPC, 4.4%; 95% CI, 2.2–6.8%), there was a decline in the relative use of open procedures from 98.1% in 2000 to 92.3% in 2011 (AAPC, −0.4%; 95% CI, −0.7 to −0.1%; Fig 2). The annual volume of MIS procedures increased from 3 in 2000 to 21 in 2011 (AAPC, 11.6%; 95% CI, 5.0–18.6%). Similarly, the proportion of MIS procedures among all patients undergoing a liver operation for a benign liver tumor increased from 1.9% in 2000 to 7.7% in 2011 (AAPC, 7.4%; 95% CI, 1.9–13.3%; Fig 2). After excluding liver ablation, the total number of MIS procedures increased from 31 in period 2000–2003 to 37 in period 2008–2011 (AAPC, 5.0; 95% CI, −4.7 to 15.6; P = .35). The total number of partial hepatectomies increased from 54 in 2000 to 142 in 2011 (AAPC, 7.9%; 95% CI, 5.3–10.6%); similarly, the proportion of cases that were characterized as a partial hepatectomy also increased from 34.6% in 2000 to 52.2% in 2011 (AAPC, 3.1%; 95% CI, 1.3–4.9%). In contrast, the number of hepatic wedge resections decreased from 18 in 2000 to 14 in 2011 (AAPC, −4.6%; 95% CI, −8.0 to −1.0%) as reflected in the relative decline from 11.5% in 2000 to 5.1% in 2011 (AAPC, −8.5%; 95% CI, −10.4 to −6.5%). There was no trend in the use of hepatic lobectomy (AAPC, 0.9%; 95% CI, −3.1 to 5.1%) or the relative use of hepatic lobectomy (AAPC, −1.9%; 95% CI, − 4.5 to 0.6%) over the study period.
Fig 1.
Trends in (A) annual volume and (B) proportion of hepatic resection for benign liver tumors. (Color illustration of this figure is available online.)
Fig 2.
Trends in annual volume of (A) open procedures and (B) minimally invasive surgery (MIS) procedures and proportion of (C) open procedure and (D) MIS procedures of hepatic resection for benign liver tumors.
Trends among various subgroups
Relative utilization of open and MIS were assessed in various subgroups (Table III). Although there was a decline in the relative use of open versus MIS procedures across all subgroups, the increase in relative utility of MIS was most pronounced among younger patients (age < 65 years [AAPC, 8.4%; 95% CI, 0.1–17.3%]). Also, relative use of an open approach decreased most among teaching (AAPC, −0.9%; 95% CI, −1.7 to −0.2%) and urban (AAPC, −0.9%; 95% CI, −1.5 to −0.2%) hospitals.
Table III.
Temporal trends in liver resection for benign liver tumors stratified by patients and hospital characteristics, 2000–2011
| Characteristic | Period 1 (2000–2003) | Period 2 (2004–2007) | Period 3 (2008–2011) | 2000–2011 AAPC | 95% CI |
|---|---|---|---|---|---|
| Age <65 (n = 1,893) | |||||
| Open | 592 (96.1) | 730 (95.4) | 869 (93.8) | −0.3 | (−0.8, 0.2) |
| MIS | 24 (3.9) | 35 (4.6) | 57 (6.2) | 8.4* | (0.1, 17.3) |
| Female (n = 2,157) | |||||
| Open | 537 (96.4) | 673 (94.9) | 834 (93.6) | −0.4 | (−0.9, 0.1) |
| MIS | 20 (3.6) | 36 (5.1) | 57 (6.4) | 9.7 | (−0.1, 20.5) |
| Teaching hospital (n = 1,519) | |||||
| Open | NA | 542 (95.3) | 885 (93.2) | −0.9* | (−1.7, −0.2) |
| MIS | NA | 27 (4.8) | 65 (6.8) | 14.9 | (−0.6, 32.8) |
| Urban hospital (n = 1,656) | |||||
| Open | NA | 598 (94.8) | 954 (93.1) | −0.9* | (−1.5, −0.2) |
| MIS | NA | 33 (5.2) | 71 (6.9) | 11.3 | (−2.9, 27.6) |
AAPC, Annual percent change; MIS, minimally invasive surgery;
P<0.05.
DISCUSSION
Benign liver tumors are common lesions that may be present in up to 20% of the population at autopsy.6,30 With the expanding use of abdominal imaging, benign liver tumors are being identified increasingly31 and continue to represent a management challenge.22 Benign liver tumors are classified into solid or cystic tumors according to features on radiographic imaging.1 The most common solid benign liver tumors include hemangiomas, focal nodular hyperplasia, and hepatic adenoma, whereas simple cysts represent the most common nonsolid lesion.32 Although benign liver tumors can have varied natural histories and need to be managed using an individualized approach, the main indication for operative resection of most benign liver tumors is the presence of significant clinical symptoms or potential malignant transformation.33–36 Although malignant transformation of benign hepatic tumors is an uncommon phenomenon, it can occur. In particular, patients with multiple, large adenomas have a greater chance for malignant transformation.37,38 Most previous studies on the topic of operative management of benign liver tumors come from a single-institution or cases series derived from multiple academic centers.39,40 These data may not reflect the management of benign liver lesions at the national, population-based level. In addition, no previous study has specifically examined trends in the utilization of surgery for benign liver tumors as an operative indication. The current study is important because we utilized a nationally representative cohort to assess both the overall use of operative procedures for benign liver tumors as well as the relative utility of MIS in a nationally representative cohort. Specifically, we demonstrated that the volume of operative procedures for benign liver tumors has increased significantly over the past decade. In addition, the relative use of MIS has increased compared with open procedures in managing benign liver tumors. Although surgery for benign liver tumors has increased, morbidity and mortality remained the same over time with up to one-third of patients experiencing a complication.
Over the time periods examined, there was a significant increase in the total volume of operative procedures performed for benign liver tumors. Several investigators have previously suggested that an increasing proportion of patients with benign liver tumors receive therapeutic procedures.41,42 Unlike previous work, data from the current study specifically quantified the increase in operative procedures for benign liver tumors using a nationally representative dataset.19,43,44 In doing this, we noted that the number of hepatic procedures performed for patients with benign liver tumors increased by >50% from 2000 to 2011 with an AAPC of 5.8% (Fig 1). Furthermore, the increase in operations was most attributable to an increase in the proportion of patients who underwent a partial hepatectomy (from 34.6% in 2000 to 52.2% in 2011). The reason for the increase in the utilization of operative intervention for benign liver tumors is clearly multifactorial and was beyond the scope of the current study. Possible explanations include increased detection of benign lesions with the ubiquitous use of cross-sectional imaging, as well as the proliferation of the MIS approach to hepatopancreatobiliary (HPB) surgery.2,27 Other investigators have suggested that the increasing acceptance of laparoscopy may, in part, explain the increase use of the operative approach for benign liver tumors.45 For example, in a review by Toro et al,42 the authors reported that laparoscopy had increased the use of hepatic resection for benign liver tumors. Our data were consistent with this finding; there was a marked increase in the use of MIS procedures relative to open procedures for operative management of benign liver tumors over the time periods examined. Several investigators have reported that an MIS approach can be performed safely for even large, complicated, benign lesions.23,46–48 Whether a less invasive operative approach should change the indication for surgery remains, however, controversial. Although some surgeons have advocated that an MIS approach should allow for the expansion of the indications for surgery of benign liver tumors, others have disagreed.21 Data from the current study demonstrate that the expansion of the MIS approach parallels the increase in surgery for benign liver tumors, suggesting that the two may indeed be related. Although we cannot comment on whether the indications for operative management were appropriate or not, the data serve to emphasize that HPB surgeons need to remain vigilant regarding the indications for operations, regardless of advances in MIS.
Interestingly, although we noted that the overall utilization of surgery for benign tumors increased over the last decade, the incidence of perioperative complications remained the same. In fact, although mortality was very low at <1%, the incidence of perioperative complication was about 30% or 1 in 3 patients. These data are consistent with other reports that have noted that hepatic resection of benign liver tumors can be performed safely with a low mortality, but with a modest amount of morbidity.19,49 In fact, most large series that have reported on perioperative outcomes for patients undergoing HPB operative procedures for either a benign or malignant indication have reported a morbidity in the range of 30–40%.27,50 In addition, patients undergoing an operation for a benign procedure had a median hospital stay of 5 days, which again was comparable with previous data on duration of stay reported for liver resection for malignant indication.22 Of note, in the current study, the incidence of complications, as well as median duration of stay, was lower among patients with benign liver tumors who underwent an MIS versus open approach. These population-based data were consistent with previously published data from highly specialized academic centers, which have reported lower morbidity with MIS versus open operative procedures.45,51,52 Collectively, the data suggest that surgery for benign liver tumors was associated with a low mortality, but a modest morbidity.
Avoiding unnecessary operations for patients with asymptomatic benign liver tumors should be the standard, even though the MIS liver surgery is safe and feasible.53 Most benign liver lesions can be managed appropriately with simple observation. In fact, as imaging becomes more sensitive and specific for hepatic masses, asymptomatic benign appearing lesions can be followed with greater confidence.54 The risk of hemorrhage, rupture, or malignant transformation is very low with the exception of hepatic adenomas, which have an increased risk of these complications based on their size.55 On occasion, the distinction between a benign and malignant lesion can be difficult to discern based on cross-sectional imaging alone, leading to diagnostic uncertainty and the need for resection. However, the indication for surgery of most benign lesions largely involves the presence of severe or progressive symptoms. Kneuertz et al20 reported on 255 patients who underwent surgery for benign liver lesions and who completed a quality-of-life survey. In that series, the most common presenting symptoms included abdominal pain (70.9%), nausea/vomiting (5.0%), and early satiety (5.0%). The operation involved less than a hemihepatectomy (68.2%), and a laparoscopic approach was utilized in 40.8% of patients. After the operation, the proportion of patients who reported moderate to extreme pain decreased from 46.9% to 15.6% and 6.8% at 6 months and 1-year, respectively (P < .001). Patient self-reported mean pain scores also decreased over time (1.65 [preoperative] vs 0.63 [6 months] vs 0.28 [1 year]; P <.001). Patients with ‘‘moderate to extreme’’ pain preoperatively were more likely to report an improvement in pain scores (odds ratio, 1.96; 95% CI, 1.05–3.66; P = .03). As such, the authors concluded that only those patients with significant preoperative symptoms derive the most benefit from operative intervention.
Our study had several limitations that should be considered. First, benign liver tumors and comorbidities were identified using ICD-9-CM codes in the NIS database. As such, we could not stratify the analyses by more specific categories of benign liver tumors, such as hemangioma, focal nodular hyperplasia, and hepatic adenoma. Although the NIS provides data to quantify nationwide use of inpatient procedures, the NIS does not allow quantification of outpatient procedures for liver benign tumors. As such, those patients who may have undergone a minor procedure such as laparoscopic cyst fenestration may have been omitted; however, the number of such cases is likely to be low. Although the number of MIS cases was relatively small, MIS surgery for benign liver disease not the main focus of the current study. Rather, we sought to define overall surgical trends in the use of operative intervention for benign liver lesions, highlighting the relative use of an MIS approach. Finally, we were unable to characterize the reason or ‘‘appropriateness’’ of any procedure for a benign indication. However, the purpose of the current study was to define temporal trends in nationwide operative procedures associated with benign liver tumors in the United States, irrespective of the clinical indication.
In conclusion, the overall volume of operative procedures for the management of liver benign tumors has increased substantially over the past decade. There has been a relative shift away from open procedures and toward MIS procedures. Future studies are warranted to assess the indication and therapeutic guidelines for benign liver tumors so as to define which patients are most likely to benefit from an operative approach.
Footnotes
Conflict of interest: none.
References
- 1.Mortele KJ, Ros PR. Benign liver neoplasms. Clin Liver Dis. 2002;6:119–45. doi: 10.1016/s1089-3261(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 2.Cristiano A, Dietrich A, Spina JC, Ardiles V, de Santibanes E. Focal nodular hyperplasia and hepatic adenoma: current diagnosis and management. Updates Surg. 2014;66:9–21. doi: 10.1007/s13304-013-0222-3. [DOI] [PubMed] [Google Scholar]
- 3.Ishak KG, Rabin L. Benign tumors of the liver. Med Clin North Am. 1975;59:995–1013. doi: 10.1016/s0025-7125(16)31998-8. [DOI] [PubMed] [Google Scholar]
- 4.Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183–8. doi: 10.1136/jcp.39.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vachha B, Sun MR, Siewert B, Eisenberg RL. Cystic lesions of the liver. Am J Roentgenol. 2011;196:W355–66. doi: 10.2214/AJR.10.5292. [DOI] [PubMed] [Google Scholar]
- 6.Soares KC, Arnaoutakis DJ, Kamel I, Anders R, Adams RB, Bauer TW, et al. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J Am Coll Surg. 2014;218:119–28. doi: 10.1016/j.jamcollsurg.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke DL, Currie EJ, Madhavan KK, Parks RW, Garden OJ. Hepatic resection for benign non-cystic liver lesions. HPB (Oxford) 2004;6:115–9. doi: 10.1080/13651820410026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanless IR. Benign liver tumors. Clin Liver Dis. 2002;6:513–26. doi: 10.1016/s1089-3261(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 9.Chiche L, Adam JP. Diagnosis and management of benign liver tumors. Semin Liver Dis. 2013;33:236–47. doi: 10.1055/s-0033-1351779. [DOI] [PubMed] [Google Scholar]
- 10.Al-Hawary MM, Haddad MC, Hourani MH, Birjawi GA, Al-Kutoubi AO. Imaging of common benign solid liver tumors. J Med Liban. 2002;50:237–46. [PubMed] [Google Scholar]
- 11.Al-Hawary MM, Ammouri NF, Al-Shahed MS, Sammak BM, Haddad MC. Imaging of uncommon and rare benign solid liver tumors. J Med Liban. 2003;51:38–50. [PubMed] [Google Scholar]
- 12.Belghiti J, Cauchy F, Paradis V, Vilgrain V. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol. 2014;11:737–49. doi: 10.1038/nrgastro.2014.151. [DOI] [PubMed] [Google Scholar]
- 13.Paradis V. Benign liver tumors: an update. Clin Liver Dis. 2010;14:719–29. doi: 10.1016/j.cld.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Rebouissou S, Bioulac-Sage P, Zucman-Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol. 2008;48:163–70. doi: 10.1016/j.jhep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Liau SS, Qureshi MS, Praseedom R, Huguet E. Molecular pathogenesis of hepatic adenomas and its implications for surgical management. J Gastrointest Surg. 2013;17:1869–82. doi: 10.1007/s11605-013-2274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in he-patocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–24. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 17.Shaked O, Siegelman ES, Olthoff K, Reddy KR. Biologic and clinical features of benign solid and cystic lesions of the liver. Clin Gastroenterol Hepatol. 2011;9:547–562. e1–e4. doi: 10.1016/j.cgh.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim S, Chen CL, Wang SH, Lin CC, Yang CH, Yong CC, et al. Liver resection for benign liver tumors: indications and outcome. Am J Surg. 2007;193:5–9. doi: 10.1016/j.amjsurg.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, De-Matteo RP, Fong Y, et al. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808–13. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 20.Kneuertz PJ, Marsh JW, de Jong MC, Covert K, Hyder O, Hirose K, et al. Improvements in quality of life after surgery for benign hepatic tumors: results from a dual center analysis. Surgery. 2012;152:193–201. doi: 10.1016/j.surg.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Kalil AN, Mastalir ET. Laparoscopic hepatectomy for benign liver tumors. Hepatogastroenterology. 2002;49:803–5. [PubMed] [Google Scholar]
- 22.Kammula US, Buell JF, Labow DM, Rosen S, Millis JM, Posner MC. Surgical management of benign tumors of the liver. Int J Gastrointest Cancer. 2001;30:141–6. doi: 10.1385/IJGC:30:3:141. [DOI] [PubMed] [Google Scholar]
- 23.Singhal A, Huang Y, Kohli V. Laparoscopic liver resection for benign and malignant liver tumors. Hepatobiliary Pancreat Dis Int. 2011;10:38–42. doi: 10.1016/s1499-3872(11)60005-2. [DOI] [PubMed] [Google Scholar]
- 24.Katkhouda N, Mavor E, Gugenheim J, Mouiel J. Laparoscopic management of benign cystic lesions of the liver. J Hepatobiliary Pancreat Surg. 2000;7:212–7. doi: 10.1007/s005340050178. [DOI] [PubMed] [Google Scholar]
- 25.Fallahzadeh MK, Zibari GB, Hamidian Jahromi A, Chu Q, Shi R, Shokouh-Amiri H. Laparoscopic versus open liver resection for benign and malignant solid liver tumors: a case-matched study. J Laparoendosc Adv Surg Tech A. 2013;23:908–11. doi: 10.1089/lap.2013.0372. [DOI] [PubMed] [Google Scholar]
- 26.HCUP Nationwide Inpatient Sample (NIS) [cited 2014 Sep 1]. Available from: http://www.hcup-us.ahrq.gov/nisoverview.Jsp.
- 27.Ejaz A, Sachs T, He J, Spolverato G, Hirose K, Ahuja N, et al. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections using the Nationwide Inpatient Sample. Surgery. 2014;156:538–47. doi: 10.1016/j.surg.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosales-Velderrain A, Bowers SP, Goldberg RF, Clarke TM, Buchanan MA, Stauffer JA, et al. National trends in resection of the distal pancreas. World J Gastroenterol. 2012;18:4342–9. doi: 10.3748/wjg.v18.i32.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Semelka RC, Sofka CM. Hepatic hemangiomas. Magn Reson Imaging Clin N Am. 1997;5:241–53. [PubMed] [Google Scholar]
- 31.Martin DR, Semelka RC. Imaging of benign and malignant focal liver lesions. Magn Reson Imaging Clin N Am. 2001;9:785–802. vi–vii. [PubMed] [Google Scholar]
- 32.Rodriguez-Pelaez M, Menendez De Llano R, Varela M. Benign liver tumors. Gastroenterol Hepatol. 2010;33:391–7. doi: 10.1016/j.gastrohep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Colli A, Fraquelli M, Massironi S, Colucci A, Paggi S, Conte D. Elective surgery for benign liver tumours. Cochrane Database Syst Rev. 2007:CD005164. doi: 10.1002/14651858.CD005164.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibbs JF, Litwin AM, Kahlenberg MS. Contemporary management of benign liver tumors. Surg Clin North Am. 2004;84:463–80. doi: 10.1016/j.suc.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Ramia JM, Muffak K, Villar J, Garrote D, Ferron JA. Benign solid liver tumors. Cir Esp. 2005;77:247–53. doi: 10.1016/s0009-739x(05)70848-4. [DOI] [PubMed] [Google Scholar]
- 36.Choi BY, Nguyen MH. The diagnosis and management of benign hepatic tumors. J Clin Gastroenterol. 2005;39:401–12. doi: 10.1097/01.mcg.0000159226.63037.a2. [DOI] [PubMed] [Google Scholar]
- 37.Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–705. doi: 10.1053/j.gastro.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 38.Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994;129:712–7. doi: 10.1001/archsurg.1994.01420310044007. [DOI] [PubMed] [Google Scholar]
- 39.Ardito F, Tayar C, Laurent A, Karoui M, Loriau J, Cherqui D. Laparoscopic liver resection for benign disease. Arch Surg. 2007;142:1188–93. doi: 10.1001/archsurg.142.12.1188. [DOI] [PubMed] [Google Scholar]
- 40.Reddy KR, Kligerman S, Levi J, Livingstone A, Molina E, Franceschi D, et al. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173–8. [PubMed] [Google Scholar]
- 41.Mezhir JJ, Fourman LT, Do RK, Denton B, Allen PJ, D’Angelica MI, et al. Changes in the management of benign liver tumours: an analysis of 285 patients. HPB (Oxford) 2013;15:156–63. doi: 10.1111/j.1477-2574.2012.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toro A, Gagner M, Di Carlo I. Has laparoscopy increased surgical indications for benign tumors of the liver? Langen-becks Arch Surg. 2013;398:195–210. doi: 10.1007/s00423-012-1012-y. [DOI] [PubMed] [Google Scholar]
- 43.Kochin IN, Miloh TA, Arnon R, Iyer KR, Suchy FJ, Kerkar N. Benign liver masses and lesions in children: 53 cases over 12 years. Isr Med Assoc J. 2011;13:542–7. [PubMed] [Google Scholar]
- 44.Erdogan D, Busch OR, Gouma DJ, van Gulik TM. Morbidity and mortality after liver resection for benign and malignant hepatobiliary lesions. Liver Int. 2009;29:175–80. doi: 10.1111/j.1478-3231.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 45.Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, et al. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. 2008;22:38–44. doi: 10.1007/s00464-007-9527-y. [DOI] [PubMed] [Google Scholar]
- 46.Abu Hilal M, Di Fabio F, Teng MJ, Godfrey DA, Primrose JN, Pearce NW. Surgical management of benign and indeterminate hepatic lesions in the era of laparoscopic liver surgery. Dig Surg. 2011;28:232–6. doi: 10.1159/000321891. [DOI] [PubMed] [Google Scholar]
- 47.Cai XJ, Huang H, Yu H, Liang X, Huang DY, Zheng XY, et al. Laparoscopic liver resection for benign liver tumors. Zhonghua Yi Xue Za Zhi. 2004;84:1698–700. [PubMed] [Google Scholar]
- 48.Yoon YS, Han HS, Choi YS, Lee SI, Jang JY, Suh KS, et al. Total laparoscopic left lateral sectionectomy performed in a child with benign liver mass. J Pediatr Surg. 2006;41:e25–8. doi: 10.1016/j.jpedsurg.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 49.Petri A, Hohn J, Kokai EL, Savanya GK, Lazar G. Surgery of benign liver tumors: indications for treatment: twenty years’ experience. Hepatogastroenterology. 2008;55:592–5. [PubMed] [Google Scholar]
- 50.Hyder O, Marsh JW, Salem R, Petre EN, Kalva S, Liapi E, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20:3779–86. doi: 10.1245/s10434-013-3127-y. [DOI] [PubMed] [Google Scholar]
- 51.Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, et al. Laparoscopic liver resection of benign liver tumors. Surg Endosc. 2003;17:23–30. doi: 10.1007/s00464-002-9047-8. [DOI] [PubMed] [Google Scholar]
- 52.Gigot JF, Hubert C, Banice R, Kendrick ML. Laparoscopic management of benign liver diseases: where are we? HPB (Oxford) 2004;6:197–212. doi: 10.1080/13651820410023950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terkivatan T, Hussain SM, De Man RA, Ijzermans JN. Diagnosis and treatment of benign focal liver lesions. Scand J Gastroenterol Suppl. 2006:102–15. doi: 10.1080/00365520600664391. [DOI] [PubMed] [Google Scholar]
- 54.Albiin N. MRI of focal liver lesions. Curr Med Imaging Rev. 2012;8:107–16. doi: 10.2174/157340512800672216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16:640–8. doi: 10.1245/s10434-008-0275-6. [DOI] [PubMed] [Google Scholar]