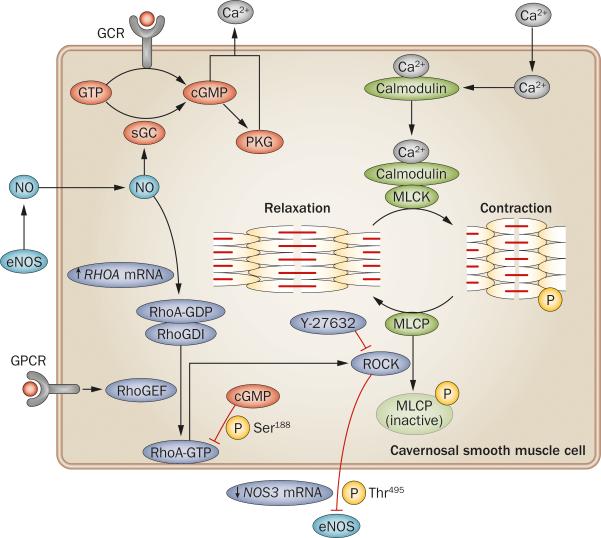
Figure 1.
Pathways of contraction and relaxation in cavernosal smooth-muscle cells. Ca2+ influx into cells increases intracellular Ca2+ that is available to bind to calmodulin. This binding causes a conformational change, enabling complexing with MLCK, with subsequent phosphorylation of the myosin–actin complex resulting in smooth muscle contraction and a flaccid penis. The Rho pathway is initiated by GPCR agonist binding, which activates RhoGEF, facilitating RhoA–GDP conversion to RhoA–GTP, and dissociation from inhibitory RhoGDI. RhoA–GTP relocates to the plasma membrane, where it binds to ROCK, facilitating autophosphorylation of ROCK that enhances its ability to phosphorylate and deactivate MLCP, augmenting the contractile response of the cell to Ca2+. Relaxation is largely mediated by cGMP. Conversion of GTP to cGMP is mediated by diffusion of NO into the cell and by GCR activation. cGMP facilitates Ca2+ efflux by activating PKG and cGMP-gated ion channels. Interactions between the pathways are complex. NO can increase expression of RhoA, but cGMP causes phosphorylation of RhoA, preventing interaction with ROCK. ROCK can phosphorylate and inhibit eNOS, and reduce eNOS expression. Abbreviations: cGMP, cyclic GMP; eNOS, endothelial nitric oxide synthase; GCR, guanylyl cyclase receptor; GPCR, G-protein-coupled receptor; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; NO, nitric oxide; PKG, protein kinase G; RhoGDI, RhoA–GDP dissociation inhibitor; RhoGEF, Rho guanine exchange factor; ROCK, Rho-associated protein kinase; sGC, soluble guanylyl cyclase; Y-27632, (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide.