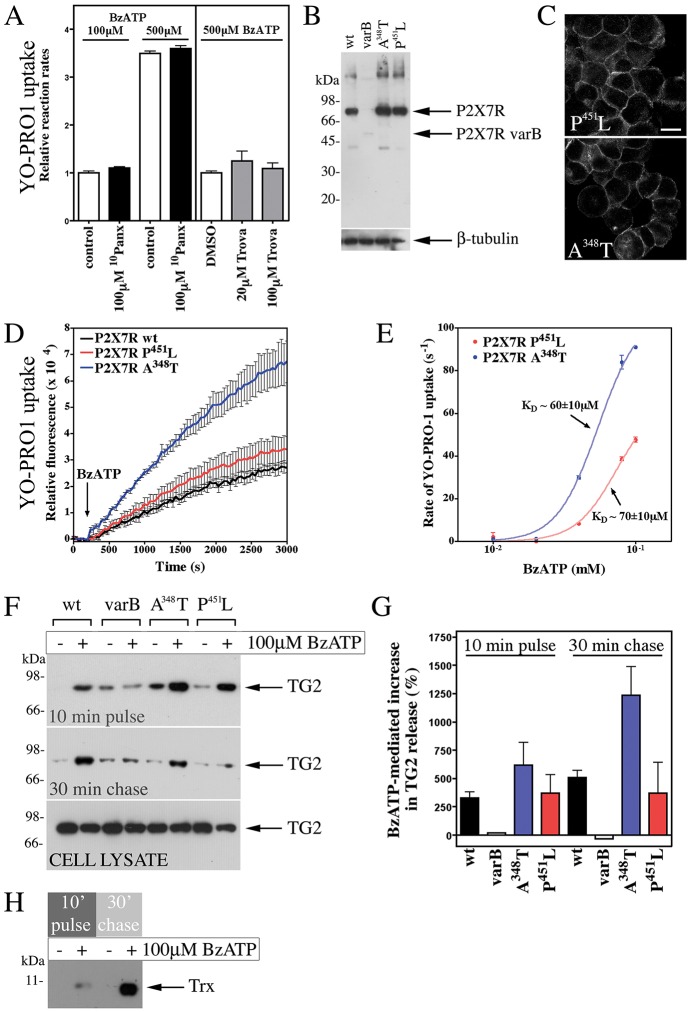
Fig. 7.
P2X7R-mediated membrane pore formation is required for TG2 externalization. (A) P2X7R-mediated pore formation is pannexin independent. P2X7R cells were pre-treated with 10Panx or trovafloxacin (Trova) as indicated, and then stimulated with BzATP in PSS with respective inhibitors, YO-PRO1 and 0.9 mM Ca2+. Results are given as initial rates of dye uptake relative to control (mean±s.e.m.; n=3). Pannexin inhibitors did not affect dye uptake, neither at limiting nor saturating agonist concentration. (B,C) Characterization of expression of mutant P2X7Rs. Extracts of cells stably expressing wild-type (wt), A348T or P451L P2X7R, or the P2X7R variant B (varB) were analyzed by western blotting with antibodies against the P2X7R extracellular domain and β-tubulin, as a loading control (B). Membrane localization of receptor was confirmed by immunocytochemistry (C; compare to Fig. S2B). Images reflect an optical section acquired by confocal microscopy. Scale bar: 12.5 µm. (D,E) Pore formation is enhanced in cells expressing P2X7R A348T. YO-PRO1 uptake following stimulation of cells with 100 µM BzATP is shown as mean±s.e.m. (n=3) fluorescence (D). Comparison of initial rate of YO-PRO1 uptake for P2X7R-A348T- and P451L-expressing cells highlights increased pore activity for P2X7R A348T but unchanged ligand regulation (E). Results are mean±s.d. (n=2). (F–H) TG2 export correlates with receptor pore activity. TG2-transfected cells expressing P2X7R variants were stimulated with BzATP for 10 min, and chased in agonist-free medium. Conditioned media were analyzed by western blotting for TG2 (F), and results (mean±s.e.m., n=3) quantified by densitometry (G). Note, cell lysates confirm comparable TG2 expression levels in different cell lines (F). For thioredoxin-1 (Trx) detection, media (P2X7R cells) were analyzed by western blotting after separation in 16% SDS-PAGE Tricine gels (H).