Abstract
We investigated prevalence of lymphogranuloma venereum (LGV) among men who have sex with men who were tested for chlamydia at 12 clinics in the United Kingdom during 10 weeks in 2012. Of 713 men positive for Chlamydia trachomatis, 66 (9%) had LGV serovars; 15 (27%) of 55 for whom data were available were asymptomatic.
Keywords: Lymphogranuloma venereum, Chlamydia trachomatis, bacteria, sexually transmitted infections, sexually transmitted diseases, men who have sex with men, bacterial screening, asymptomatic, United Kingdom, serovars, MSM, LGV, CT, STI, STD
Lymphogranuloma venereum (LGV) is a sexually transmitted infection (STI) caused by the L1, L2, and L3 serovars of Chlamydia trachomatis (CT). An LGV outbreak among men who have sex with men (MSM) first reported in the Netherlands in 2003 has since spread across other industrialized countries (1). Cases are typically seen among white, HIV-positive MSM who report unprotected anal intercourse, other high-risk behaviors, and STI co-infection and who commonly have symptoms of proctitis (i.e., rectal pain, rectal discharge, bloody stools, constipation, and tenesmus) (2).
The United Kingdom now has the largest documented outbreak of LGV among MSM worldwide (3,4). Infection control in England has relied on CT DNA typing and treatment of symptomatic MSM who have CT-positive rectal infections and their contacts, as well as health promotion. These measures were supported by a large prospective study in the United Kingdom during 2006–2007 that reported <6% of LGV CT infections were asymptomatic (5). However, studies in the Netherlands and Germany, and a smaller UK study, have reported higher proportions (17%–53%) of asymptomatic infection (6–8). We reinvestigated the prevalence of asymptomatic LGV CT infection among MSM in the United Kingdom to assess whether it may be sustaining the current epidemic.
The Study
In the UK, STI clinics are open access and provide free testing and treatment. Regular STI and HIV screening is encouraged for sexually active MSM with or without symptoms (9). A full medical and sexual history are recorded for all patients, and a physical examination is done for those with symptoms.
Twelve UK STI clinics participated; all serve cities with large MSM populations and routinely screen MSM for CT by examining urine or swab samples of the pharynx, urethra, and rectum (either clinician-obtained or self-taken) according to UK guidelines (10). All MSM tested for CT during September 24–December 7, 2012, were included except those who had received antibiotic drugs during the previous 6 weeks.
More than 10,000 CT tests were performed during the study period. Local laboratories performed routine testing for CT and referred all positive specimens from study participants to the Sexually Transmitted Bacteria Reference Unit of the national reference laboratory in London to test for LGV. At the reference laboratory, all specimens underwent extraction by using the Roche MagNA Pure LC extractor (Roche Diagnostics, Indianapolis, IN, USA), then CT confirmation by using a plasmid targeted real-time PCR and an LGV-specific real-time PCR targeting the pmpH deletion on the RotorGene (QIAGEN, Valencia, CA, USA). Details of the LGV reference service were previously published (11).
Clinical data for symptoms were submitted for all study participants to Public Health England (PHE) through a secure web portal (Technical Appendix Figure). Patients reporting symptoms at first medical examination or follow-up were defined as symptomatic. Those with no symptoms at first examination or follow-up were defined as asymptomatic. Additional clinical data were available from the national anonymized patient-level electronic surveillance system (the Genitourinary Medicine Clinic Activity Dataset [GUMCADv2]), which records all tests and diagnoses in STI clinics in England (12).
PHE has authority to collect anonymized patient-level data for public health monitoring and infection control. The study was reviewed in PHE’s research and development office and deemed to fit this criterion.
We used univariable and multivariable logistic regression modeling in STATA version 13.1 (StataCorp LP, College Station, TX, USA) to investigate risk factors associated with LGV versus non-LGV CT infection and asymptomatic versus symptomatic LGV. A clinic in Glasgow, Scotland, was excluded from risk factor analyses because it does not report GUMCADv2 (Figure).
Figure.
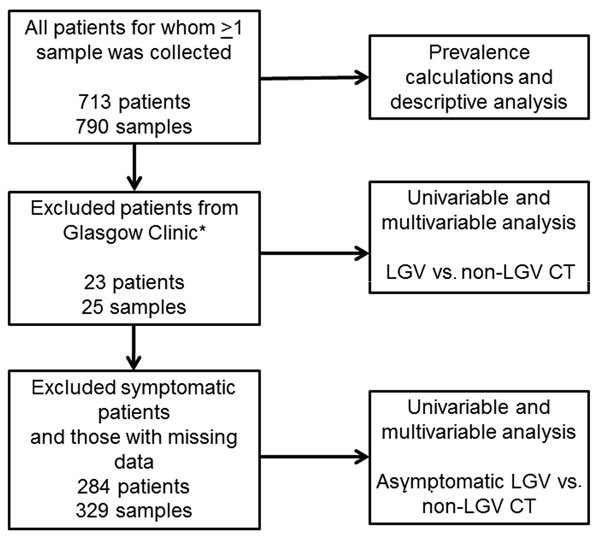
Data analysis flowchart for univariable and multivariable analyses of symptomatic lymphogranuloma venereum (LGV) versus non-LGV Chlamydia trachomatis (CT) infection (Table 1) and asymptomatic LGV versus non-LGV CTinfection (Table 2) in men who have sex with men, United Kingdom*Patients from Glasgow were excluded from risk factor analyses because they do not routinely report to the Genitourinary Medicine Clinic Activity Dataset.
During the study period, 921 eligible specimens were received for DNA typing. On confirmatory testing, 90 (10%) specimens were CT negative, 36 (4%) inhibitory, and 1 (0.1%) equivocal; 4 (0.4%) were not tested. CT infection was confirmed in 790 specimens from 713 patients; these specimens then underwent DNA typing. Overall, we found 69 (9%) LGV CT–positive specimens from 66 (9%) patients and 721 (91%) non-LGV CT–positive specimens from 647 (91%) patients (Table 1). Co-infection with LGV CT at 1 anatomic site and non-LGV CT at a different site was found in 4/713 (0.6%) patients. Clinical data coordinating with the symptom checklist and GUMCADv2 were available for 95% (680/713) and 87% (603/690) of CT-positive patients, respectively. During the study period, GUMCADv2 recorded 1,097 CT diagnoses among 10,143 MSM screened at the 11 STI clinics in England, showing an estimated CT prevalence of 10.8%.
Table 1. Descriptive, univariable, and multivariable analysis of patients diagnosed with symptomatic versus nonsymptomatic LGV CT infection among men who have sex with men, by demographic and behavioral characteristics, United Kingdom*.
| Patient characteristics |
No. (%) or median [IQR] |
|
Univariable analysis |
|
Multivariable analysis† |
|||||
| All CT |
Non-LGV CT |
LGV CT |
OR (95% CI) |
p value |
OR (95% CI) |
p value |
||||
| All patients |
713 (100) |
647 (90.7) |
66 (9.3) |
ND |
ND |
ND |
ND |
|||
| Clinic location, n = 713 | ||||||||||
| London | 563 (79.0) | 512 (79.1) | 51 (77.3) | 1 | 0.73 | |||||
| Manchester | 85 (11.9) | 75 (11.6) | 10 (15.2) | 1.34 (0.65–2.75) | ||||||
| Brighton | 42 (5.9) | 38 (5.9) | 4 (6.1) | 1.06 (0.36–3.08) | ||||||
| Glasgow‡ |
23 (3.2) |
22 (3.4) |
1 (1.5) |
|
|
|
|
|
|
|
| Infection site, n = 710 | ||||||||||
| Nonrectal | 221 (31.1) | 217 (33.7) | 4 (6.1) | 1 | 1 | |||||
| Rectal |
489 (68.9) |
427 (66.3) |
62 (93.9) |
|
7.83 (2.81–21.82) |
<0.001 |
|
10.08 (3.37–30.17) |
<0.001 |
|
| Multiple infection sites, n = 713 | ||||||||||
| No | 641 (89.9) | 581 (89.8) | 60 (90.9) | 1 | ||||||
| Yes |
72 (10.1) |
66 (10.2) |
6 (9.1) |
|
0.89 (0.37–2.15) |
0.8 |
|
|
|
|
| Symptoms present, n = 650 | ||||||||||
| No | 453 (69.7) | 438 (73.6) | 15 (27.3) | 1 | 1 | |||||
| Yes |
197 (30.3) |
157 (26.4) |
40 (72.7) |
|
7.93 (4.20–14.99) |
<0.001 |
|
13.33 (6.53–27.21) |
<0.001 |
|
| Age, y, n = 710 | 33 [27–42] | 33 [27–42] | 39 [33–46] | |||||||
| 18–24 | 108 (15.2) | 106 (16.5) | 2 (3.0) | 1 | 0.002 | |||||
| 25–34 | 276 (38.9) | 258 (40.1) | 18 (27.3) | 3.72 (0.85–16.31) | ||||||
| 35–44 | 192 (27.0) | 166 (25.8) | 26 (39.4) | 7.92 (1.84–34.15) | ||||||
|
>44 |
134 (18.9) |
114 (17.7) |
20 (30.3) |
|
9.11 (2.08–39.93) |
|
|
|
|
|
| Ethnicity, n = 603 | ||||||||||
| White | 480 (79.6) | 432 (79.3) | 48 (82.8) | 1 | 0.17 | |||||
| Black or Black British | 28 (4.6) | 27 (5) | 1 (1.7) | 0.33 (0.44–2.51) | ||||||
| Mixed | 27 (4.5) | 27 (5) | 0 | NP | ||||||
| Asian and Asian British | 21 (3.5) | 21 (3.9) | 0 | NP | ||||||
| Other ethnic groups | 23 (3.8) | 19 (3.5) | 4 (6.9) | 1.89 (0.62–5.80) | ||||||
| Unknown |
24 (4) |
19 (3.5) |
5 (8.6) |
|
2.37 (0.85–6.63) |
|
|
|
|
|
| HIV status, n = 603 | ||||||||||
| Negative | 367 (60.9) | 350 (64.2) | 17 (29.3) | 1 | 1 | |||||
| Positive |
236 (39.1) |
195 (35.8) |
41 (70.7) |
|
4.33 (2.40–7.82) |
<0.001 |
|
3.63 (1.80–7.32) |
<0.001 |
|
| No. sexual partners in previous 3 mo, n = 635 | ||||||||||
| No. partners | 3 [1–5] | 3 [1–5] | 3 [1–8] | |||||||
| 0–1 | 169 (26.6) | 153 (26.3) | 16 (29.6) | 1 | 0.26 | |||||
| 2–5 | 321 (50.6) | 299 (51.5) | 22 (40.7) | 0.66 (0.34–1.31) | ||||||
|
>6 |
145 (22.8) |
129 (22.2) |
16 (29.6) |
|
1.13 (0.54–2.36) |
|
|
|
|
|
| Concurrent sexually transmitted infection, n = 631 | ||||||||||
| No | 451 (71.5) | 406 (71) | 45 (76.3) | 1 | ||||||
| Yes |
180 (28.5) |
166 (29) |
14 (23.7) |
|
0.76 (0.41–1.42) |
0.39 |
|
|
|
|
| STI within previous 12 mo, n = 594 | ||||||||||
| No | 469 (79) | 435 (80.3) | 34 (65.4) | 1 | ||||||
| Yes | 125 (21) | 107 (19.7) | 18 (34.6) | 2.15 (1.17–3.96) | 0.01 | |||||
*n values indicate number of patients for which data were available in each category. CT, Chlamydia trachomatis; LGV, lymphogranuloma venereum; IQR, interquartile range; OR, odds ratio; ND, no data were available; NP, not performed. Blank cells indicate not applicable. †Multivariable analysis adjusted for age and location. ‡Glasgow patients were excluded from all univariable and multivariable analyses because they do not routinely report to the Genitourinary Medicine Clinic Activity Dataset.
Compared to those positive for non-LGV CT, patients with LGV CT infection were older and more likely to be symptomatic, to be HIV-positive, to have rectal infection, and to have had a previous STI diagnosis. In adjusted logistic regression analysis, symptomatic infection (adjusted odds ratio [aOR] 13.33; p<0.001), rectal infection (aOR 10.08; p<0.001) and being HIV-positive (aOR 3.63; p<0.001) remained statistically significant (Table 1).
Of those with LGV for whom data were available, 27% (15/55) overall and 22% (12/54) with rectal-only infection were asymptomatic. Study prevalence of asymptomatic LGV was 2.3% (15/650) overall and 3.8% (9/236) in HIV-positive MSM (Tables 1, 2).
Table 2. Descriptive, univariable and multivariable analysis of patients in whom asymptomatic LGV CT or asymptomatic non-LGV CT infection was diagnosed, by demographic and behavioral characteristics, United Kingdom*.
| Patient characteristics | No. (%) or median [IQR] |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| All asymptomatic CT | Asymptomatic non-LGV CT | Asymptomatic LGV | Univariable analysis |
Multivariable analysis† |
|||||
| OR (95% CI) | p value | OR (95% CI) | p value | ||||||
| All patients |
429 (100.0) |
414 (96.5) |
15 (3.5) |
|
|
|
|
||
| Clinic location, n = 429 | |||||||||
| London | 333 (77.6) | 323 (78.0) | 10 (66.7) | 1 | 0.47 | ||||
| Manchester | 54 (12.6) | 52 (12.6) | 2 (13.3) | 1.24 (0.26–5.83) | |||||
| Brighton | 26 (6.1) | 24 (5.8) | 2 (13.3) | 2.69 (0.56–12.99) | |||||
| Glasgow‡ |
16 (3.7) |
15 (3.6) |
1 (6.7) |
|
|
|
|
|
|
| Infection site, n = 427 | |||||||||
| Nonrectal | 129 (30.2) | 126 (30.6) | 3 (20.0) | 1 | |||||
| Rectal |
298 (69.8) |
286 (69.4) |
12 (80.0) |
|
1.67 (0.46–6.08) |
0.44 |
|
|
|
| Multiple infection sites, n = 429 | |||||||||
| No | 397 (92.5) | 383 (92.5) | 14 (93.3) | 1 | |||||
| Yes |
32 (7.5) |
31 (7.5) |
1 (6.7) |
|
0.95 (0.12–7.48) |
0.96 |
|
|
|
| Age, y, n = 429 | 33 [27–42] | 33 [26–42] | 38 [29–44] | | |||||
| 18–24 | 73 (17.0) | 72 (17.4) | 1 (6.7) | 1 | 0.64 | ||||
| 25–34 | 172 (40.1) | 167 (40.3) | 5 (33.3) | 2.19 (0.25–19.07) | |||||
| 35–44 | 105 (24.5) | 99 (23.9) | 6 (40.0) | 3.72 (0.43–32.59) | |||||
|
>44 |
79 (18.4) |
76 (18.4) |
3 (20.0) |
|
2.8 (0.28–27.55) |
|
|
|
|
| Ethnicity, n = 378 | |||||||||
| White | 302 (79.9) | 290 (79.5) | 12 (92.3) | 1 | 0.32 | ||||
| Black or Black British | 18 (4.8) | 18 (4.9) | 0 | NP | |||||
| Mixed | 19 (5.0) | 19 (5.2) | 0 | NP | |||||
| Asian and Asian British | 17 (4.5) | 17 (4.7) | 0 | NP | |||||
| Other ethnic groups | 13 (3.4) | 13 (3.6) | 0 | NP | |||||
| Unknown |
9 (2.4) |
8 (2.2) |
1 (7.7) |
|
3.02 (0.35–26.13) |
|
|
|
|
| HIV status, n = 378 | |||||||||
| Negative | 235 (62.2) | 231 (63.3) | 4 (30.8) | 1 | |||||
| Positive |
143 (37.8) |
134 (36.7) |
9 (69.2) |
|
3.88 (1.17–12.84) |
0.03 |
|
3.91 (0.92–16.66) |
0.06 |
| No. sexual partners in previous 3 mo, n = 401 | |||||||||
| No. partners | 3 [1–5] | 3 [1–5] | 3 [2–5] | ||||||
| 0–1 | 106 (26.4) | 103 (26.7) | 3 (20.0) | 1 | 0.87 | ||||
| 2–5 | 207 (51.6) | 198 (51.3) | 9 (60.0) | 1.41 (0.37–5.44) | |||||
|
>6 |
88 (21.9) |
85 (22.0) |
3 (20.0) |
|
1.16 (0.23–5.90) |
|
|
|
|
| Concurrent sexually transmitted infections, n = 398 | |||||||||
| No | 295 (74.1) | 283 (73.7) | 12 (85.7) | 1 | |||||
| Yes |
103 (25.9) |
101 (26.3) |
2 (14.3) |
|
0.47 (0.10–2.12) |
0.32 |
|
|
|
| STI during previous 12 mo, n = 374 | |||||||||
| No | 297 (79.4) | 291 (80.4) | 6 (50.0) | 1 | |||||
| Yes | 77 (20.6) | 71 (19.6) | 6 (50.0) | 4.1 (1.28–13.09) | 0.02 | 3.1 (0.87–10.99) | 0.08 | ||
*n values indicate number of patients for which data were available in each category. CT, Chlamydia trachomatis; LGV, lymphogranuloma venereum; No., number; IQR, interquartile range; CI, confidence interval; OR, odds ratio; NP, not performed. Blank cells indicate not applicable. †Multivariable analysis adjusted for age and location ‡Glasgow patients were excluded from all univariable and multivariable analyses because they do not routinely report to the Genitourinary Medicine Clinic Activity Dataset.
Of the 15 patients with asymptomatic LGV, 12 (80%) had rectal, 2 (13%) urethral, and 1 (7%) pharyngeal infections. All cases of asymptomatic LGV were from patients with single-site infection.
Among asymptomatic patients, those with LGV were more likely to be HIV-positive (69% vs. 31%; odds ratio 3.88; p = 0.03) and to have had an STI in the past 12 months (50% vs. 20%; odds ratio 4.1; p = 0.02) than those infected with non-LGV CT. These characteristics were only weakly associated in the adjusted analysis (aOR 3.91, p = 0.06, and aOR 3.1, p = 0.08, respectively).
Conclusions
This large multicenter case-finding study found a higher rate of asymptomatic LGV (27%) than previously reported in the United Kingdom, in agreement with studies done in Germany and the Netherlands. LGV case-patients were typically older, white, HIV-positive MSM who had a concurrent or recent STI diagnosis. Most infections were rectal; few urethral and pharyngeal infections were detected.
The number of CT infections confirmed at the reference laboratory (713) was lower than those reported to national surveillance (1,097), possibly related to differences in test sensitivity, degradation of CT DNA during transportation, or incorrect surveillance coding. No patients were excluded because of study restrictions; therefore, it is likely the study case-patients were representative of all MSM with diagnoses of CT infection in the United Kingdom.
More than one quarter of LGV cases in the United Kingdom may go undiagnosed if those who have asymptomatic chlamydial infection are not tested, as is the current strategy. Recommending that all CT-positive specimens from MSM be DNA tested for LGV serovars is unlikely to be cost-effective or feasible. However, because 3.8% of asymptomatic HIV-positive MSM had LGV (i.e., in excess of the recommended 3% prevalence threshold for CT screening [13]), inclusion of these patients in the testing algorithm, as is done in Scotland (14), may be warranted.
Whether LGV symptomatology in the United Kingdom has changed or asymptomatic cases were previously missed is unclear. Changes in screening practice or selection pressure for asymptomatic infection after treatment of persons with symptomatic infection may have contributed. Most asymptomatic patients will be treated for non-LGV CT infection, but if treatment is suboptimal, it may not prevent onward transmission (15).
An undiagnosed reservoir of CT infection is unlikely to be the sole cause of the current epidemic. High-risk sexual behavior remains a substantive challenge for control of LGV and related epidemics among MSM (3). Future public health strategies will require a combined strategy of increased testing, prompt treatment, and continued promotion of safer sexual behavior among MSM.
Technical Appendix. Symptom checklist posted on Public Health England site for detection of asymptomatic lymphogranuloma venereum infection in men who have sex with men.
Acknowledgments
Additional members of the UK LGV Case-Finding Group who contributed data: Sameena Ahmad (University Hospitals of South Manchester National Health Service [NHS] Foundation Trust, Manchester, UK) Nadia Ahmed, Patrick French (Central and North West London NHS Foundation Trust, London, UK); Sarah Alexander, Hemanti Patel, Pamela Saunders, Sinan Turkaslan (Sexually Transmitted Bacteria Reference Unit, Public Health England, Colindale, London); Tristan Childs, Stephen Duffell, Rishma Maini, Chinelo Obi, Parnam Seyan (Centre for Infectious Disease Surveillance and Control, PHE Colindale); Sara Day, Jamie Hardie, Ken McKlean, Alan McOwan, Christopher Scott, Richard Stack, Ann Sullivan (Chelsea and Westminster Hospital NHS Foundation Trust, London); Gillian Dean, Mohammed Hassan-Ibrahim, Gary Homer (Brighton and Sussex University Hospitals NHS Trust, Brighton, UK); Kirstine Eastick (Scottish Bacterial Sexually Transmitted Infections Reference Laboratory, Health Protection Scotland, Edinburgh, Scotland, UK); Laura Greaves, Maria Sampson, Merle Symonds (Barts Health NHS Trust, London); Rory Gunson, Anthony Rea, Andrew Winter (NHS Greater Glasgow and Clyde Health Board, Glasgow, Scotland, UK); Peter Horne, Daniel Krahe, Iain Reeves (Homerton University Hospital NHS Foundation Trust, London); Monica Rebec, Javier Rubio, Dawn Wilkinson (Imperial College Healthcare NHS Trust, London); Gabriel Schembri, Peter Tilston, Andrew Turner (Central Manchester University Hospitals NHS Foundation Trust); Simon Stevenson (University College London Hospitals NHS Foundation Trust); Helen Ward (Imperial College London); John White (Guy’s and St. Thomas’ NHS Foundation Trust, London).
Biography
Dr. Saxon is a consultant in sexual health and HIV in Manchester, UK. She completed this study during a joint fellowship of Public Health England and British Association of Sexual Health and HIV.
Footnotes
Suggested citation for this article: Saxon C, Hughes G, Ison C. Asymptomatic lymphogranuloma venereum in men who have sex with men, United Kingdom. Emerg Infect Dis. 2016 Dec [date cited]. http://dx.doi.org/10.3201/eid2201.141867
Additional members of the UK LGV Case-Finding Group who contributed to data collection, analysis, and manuscript review are listed at the end of this article.
References
- 1.Götz H, Nieuwenhuis R, Ossewaarde T, Thio B, van der Meijden W, Dees J. Preliminary report of an outbreak of lymphogranuloma venereum in homosexual men in the Netherlands, with implications for other countries in western Europe. Euro Surveill. 2004;8:2367. [PubMed] [Google Scholar]
- 2.Hamill M, Benn P, Carder C, Copas A, Ward H, Ison C, et al. The clinical manifestations of anorectal infection with lymphogranuloma venereum (LGV) versus non-LGV strains of Chlamydia trachomatis: a case-control study in homosexual men. Int J STD AIDS. 2007;18:472–5 . 10.1258/095646207781147319 [DOI] [PubMed] [Google Scholar]
- 3.Hughes G, Alexander S, Simms I, Conti S, Ward H, Powers C, et al. Lymphogranuloma venereum diagnoses among men who have sex with men in the UK: interpreting a cross-sectional study using an epidemic phase-specific framework. Sex Transm Infect. 2013;89:542–7. 10.1136/sextrans-2013-051051 [DOI] [PubMed] [Google Scholar]
- 4.Public Health England. Latest LGV surveillance data [cited 2015 Aug 21]. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/LGV/
- 5.Ward H, Alexander S, Carder C, Dean G, French P, Ivens D, et al. The prevalence of lymphogranuloma venereum in men who have sex with men: results of a multicentre case finding study. Sex Transm Infect. 2009;85:173–5. 10.1136/sti.2008.035311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spaargaren J, Fennema HAS, Morré SA, de Vries HJC, Coutinho RA. New lymphogranuloma venereum Chlamydia trachomatis variant, Amsterdam. Emerg Infect Dis. 2005;11:1090–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haar K, Dudareva-Vizule S, Wislinghoff H, Sailer A, Jansen K, Henrich B, et al. Lymphogranuloma venereum in men screened for pharyngeal and rectal infection, Germany. Emerg Infect Dis. 2013;19:488–92. 10.3201/eid1903.121028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annan NT, Sullivan AK, Nori A, Naydenova P, Alexander S, McKenna A, et al. Rectal chlamydia—a reservoir of undiagnosed infection in men who have sex with men. Sex Transm Infect. 2009;85:176–9. 10.1136/sti.2008.031773 [DOI] [PubMed] [Google Scholar]
- 9.Ross J, Brady M, Clutterbuck D, Doyle T, Hart G, Hughes G, et al. British Association for Sexual Health and HIV recommendations for testing for sexually transmitted infections in men who have sex with men. London: BASHH; 2014. [cited 2015 Aug 21]. http://www.bashh.org/documents/BASHH%20Recommendations%20for%20testing%20for%20STIs%20in%20MSM%20-%20FINAL.pdf
- 10.Carder C, Mercey D. Benn P on behalf of the British Association for Sexual Health and HIV clinical effectiveness group. Chlamydia trachomatis UK testing guidelines 2010. London: BASHH; 2010. [cited 2015 Aug 21]. http://www.bashh.org/documents/3352.pdf
- 11.Ward H, Martin I, Macdonald N, Alexander S, Simms I, Fenton K, et al. Lymphogranuloma venereum in the United Kingdom. Clin Infect Dis. 2007;44:26–32. 10.1086/509922 [DOI] [PubMed] [Google Scholar]
- 12.Public Health England. Genitourinary Medicine Clinic activity dataset (GUMCADv2): Guidance for clinic staff. London; PHE; 2013. [cited 2015 Aug 21]. https://www.gov.uk/genitourinary-medicine-clinic-activity-dataset-gumcadv2
- 13.Marrazzo JM, Celum CL, Hillis SD, Fine D, DeLisle S, Handsfield HH. Performance and cost-effectiveness of selective screening criteria for Chlamydia trachomatis infection in women: implications for a national chlamydia control strategy. Sex Transm Dis. 1997;24:131–41. 10.1097/00007435-199703000-00003 [DOI] [PubMed] [Google Scholar]
- 14.White J, O’Farrell N, Daniels D. 2013 UK National guideline for the management of lymphogranuloma venereum: clinical effectiveness group of the British association for sexual health and HIV (CEG/BASHH) guideline development group. Int J STD AIDS. 2013;24:593–601 . 10.1177/0956462413482811 [DOI] [PubMed] [Google Scholar]
- 15.Hathorn E, Opie C, Goold P. What is the appropriate treatment for the management of rectal Chlamydia trachomatis in men and women? Sex Transm Infect. 2012;88:352–4. 10.1136/sextrans-2011-050466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical Appendix. Symptom checklist posted on Public Health England site for detection of asymptomatic lymphogranuloma venereum infection in men who have sex with men.


