Abstract
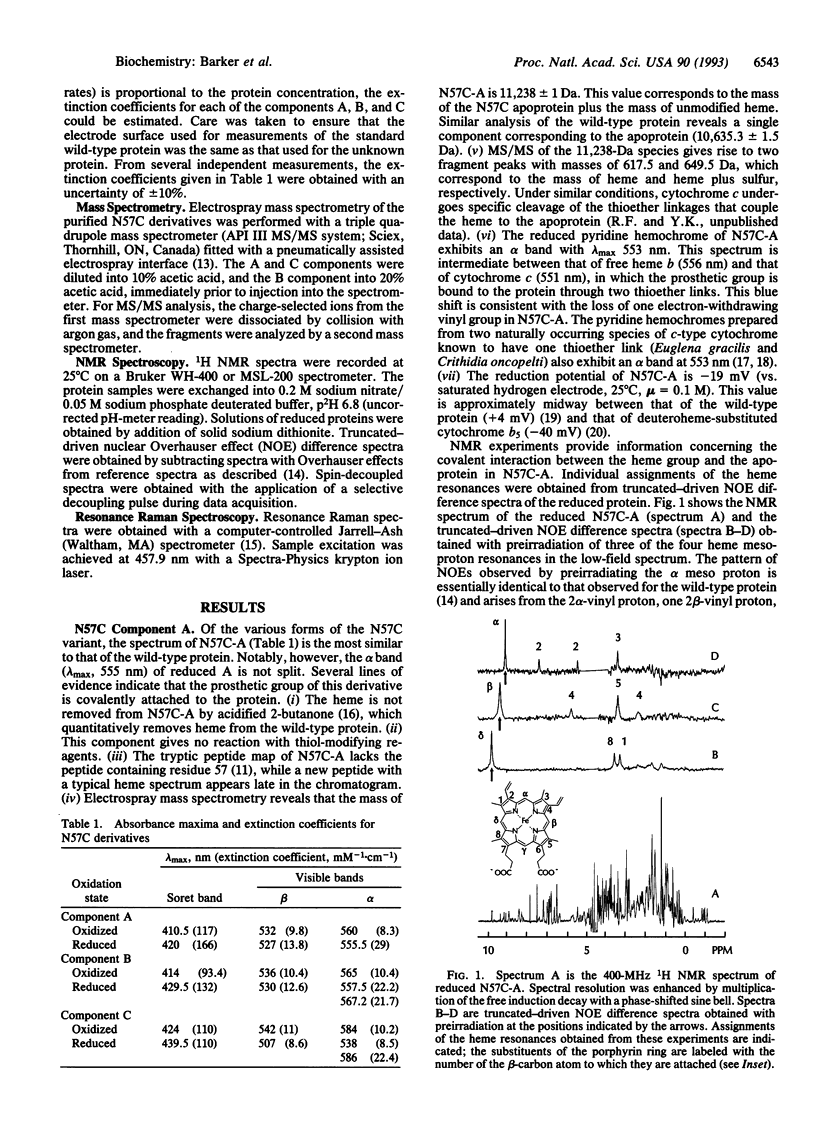
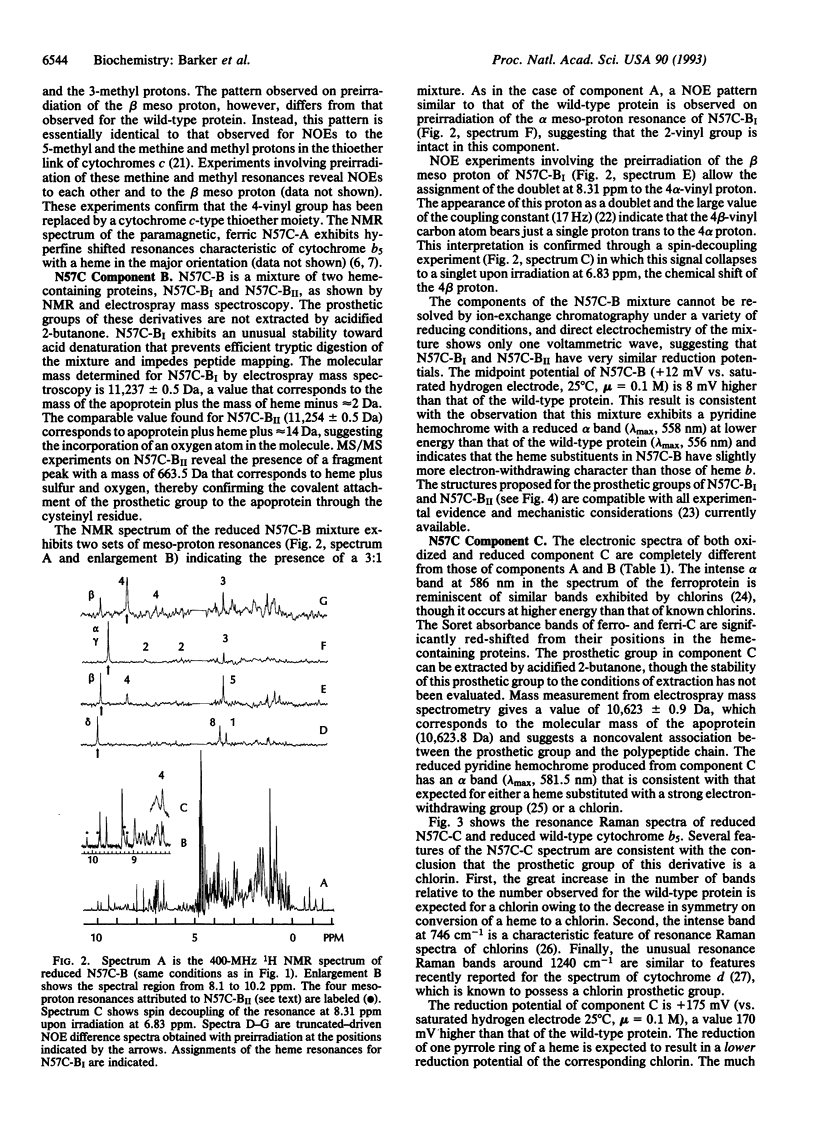
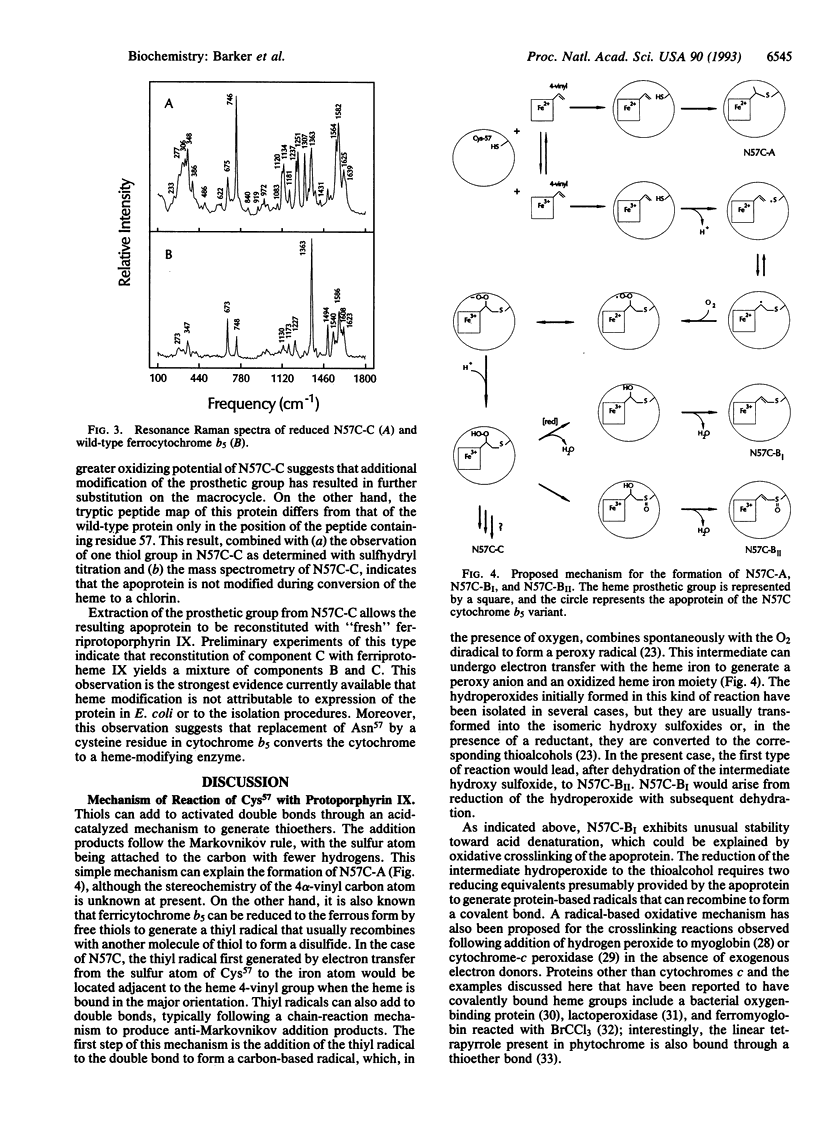
Residue Asn57 of bovine liver cytochrome b5 has been replaced with a cysteine residue, and the resulting variant has been isolated from recombinant Escherichia coli as a mixture of four major species: A, BI, BII, and C. A combination of electronic spectroscopy, 1H NMR spectroscopy, resonance Raman spectroscopy, electrospray mass spectrometry, and direct electrochemistry has been used to characterize these four major cytochrome derivatives. The red form A (E(m) = -19 mV) is found to possess a heme group bound covalently through a thioether linkage involving Cys57 and the alpha carbon of the heme 4-vinyl group. Form BI has a covalently bound heme group coupled through a thioether linkage involving the beta carbon of the heme 4-vinyl group. Form BII is similar to BI except that the sulfur involved in the thioether linkage is oxidized to a sulfoxide. The green form C (E(m) = 175 mV) possesses a noncovalently bound prosthetic group with spectroscopic properties characteristic of a chlorin. A mechanism is proposed for the generation of these derivatives, and the implications of these observations for the biosynthesis of cytochrome c and naturally occurring chlorin prosthetic groups are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby S., Barker P. D., Guo L. H., Hill H. A. Direct electrochemistry of protein-protein complexes involving cytochrome c, cytochrome b5, and plastocyanin. Biochemistry. 1990 Apr 3;29(13):3213–3219. doi: 10.1021/bi00465a010. [DOI] [PubMed] [Google Scholar]
- Barker P. D., Di Gleria K., Hill H. A., Lowe V. J. Electron transfer reactions of metalloproteins at peptide-modified gold electrodes. Eur J Biochem. 1990 May 31;190(1):171–175. doi: 10.1111/j.1432-1033.1990.tb15561.x. [DOI] [PubMed] [Google Scholar]
- CLEZY P. S., MORELL D. B. A spectroscopic study of haematin compounds in the Soret region. Biochim Biophys Acta. 1963 Apr 2;71:165–171. doi: 10.1016/0006-3002(63)90995-8. [DOI] [PubMed] [Google Scholar]
- Catalano C. E., Choe Y. S., Ortiz de Montellano P. R. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J Biol Chem. 1989 Jun 25;264(18):10534–10541. [PubMed] [Google Scholar]
- Cocco M. J., Lecomte J. T. Characterization of hydrophobic cores in apomyoglobin: a proton NMR spectroscopy study. Biochemistry. 1990 Dec 18;29(50):11067–11072. doi: 10.1021/bi00502a008. [DOI] [PubMed] [Google Scholar]
- Damaschun G., Damaschun H., Gast K., Gernat C., Zirwer D. Acid denatured apo-cytochrome c is a random coil: evidence from small-angle X-ray scattering and dynamic light scattering. Biochim Biophys Acta. 1991 Jun 24;1078(2):289–295. doi: 10.1016/0167-4838(91)90571-g. [DOI] [PubMed] [Google Scholar]
- Dumont M. E., Cardillo T. S., Hayes M. K., Sherman F. Role of cytochrome c heme lyase in mitochondrial import and accumulation of cytochrome c in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Nov;11(11):5487–5496. doi: 10.1128/mcb.11.11.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R., Konishi Y. Analysis of antibodies and other large glycoproteins in the mass range of 150,000-200,000 Da by electrospray ionization mass spectrometry. Anal Chem. 1992 Sep 15;64(18):2090–2095. doi: 10.1021/ac00042a012. [DOI] [PubMed] [Google Scholar]
- Feng Y. Q., Wand A. J., Sligar S. G. 1H and 15N NMR resonance assignments and preliminary structural characterization of Escherichia coli apocytochrome b562. Biochemistry. 1991 Aug 6;30(31):7711–7717. doi: 10.1021/bi00245a007. [DOI] [PubMed] [Google Scholar]
- Funk W. D., Lo T. P., Mauk M. R., Brayer G. D., MacGillivray R. T., Mauk A. G. Mutagenic, electrochemical, and crystallographic investigation of the cytochrome b5 oxidation-reduction equilibrium: involvement of asparagine-57, serine-64, and heme propionate-7. Biochemistry. 1990 Jun 12;29(23):5500–5508. doi: 10.1021/bi00475a013. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Kahlow M. A., Zuberi T. M., Gennis R. B., Loehr T. M. Identification of a ferryl intermediate of Escherichia coli cytochrome d terminal oxidase by resonance Raman spectroscopy. Biochemistry. 1991 Dec 10;30(49):11485–11489. doi: 10.1021/bi00113a001. [DOI] [PubMed] [Google Scholar]
- Keller R. M., Wüthrich K. Structural study of the heme crevice in cytochrome b5 based on individual assignments of the 1H-NMR lines of the heme group and selected amino acid residues. Biochim Biophys Acta. 1980 Feb 27;621(2):204–217. doi: 10.1016/0005-2795(80)90172-5. [DOI] [PubMed] [Google Scholar]
- Lee K. B., La Mar G. N., Kehres L. A., Fujinari E. M., Smith K. M., Pochapsky T. C., Sligar S. G. 1H NMR study of the influence of hydrophobic contacts on protein-prosthetic group recognition in bovine and rat ferricytochrome b5. Biochemistry. 1990 Oct 16;29(41):9623–9631. doi: 10.1021/bi00493a017. [DOI] [PubMed] [Google Scholar]
- Lin D. M., Niece R. L., Fitch W. M. The properties and amino-acid sequence of cytochrome c from Euglena gracilis. Nature. 1973 Feb 23;241(5391):533–535. doi: 10.1038/241533a0. [DOI] [PubMed] [Google Scholar]
- Loehr T. M., Keyes W. E., Pincus P. A. A computer-controlled laser Raman spectrophotometer with interactive-graphics data analysis. Anal Biochem. 1979 Jul 15;96(2):456–463. doi: 10.1016/0003-2697(79)90606-7. [DOI] [PubMed] [Google Scholar]
- Mathews F. S. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45(1):1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Mauk M. R., Mauk A. G. Crosslinking of cytochrome c and cytochrome b5 with a water-soluble carbodiimide. Reaction conditions, product analysis and critique of the technique. Eur J Biochem. 1989 Dec 22;186(3):473–486. doi: 10.1111/j.1432-1033.1989.tb15231.x. [DOI] [PubMed] [Google Scholar]
- Moore C. D., al-Misky O. N., Lecomte J. T. Similarities in structure between holocytochrome b5 and apocytochrome b5: NMR studies of the histidine residues. Biochemistry. 1991 Aug 27;30(34):8357–8365. doi: 10.1021/bi00098a012. [DOI] [PubMed] [Google Scholar]
- Nelson R. M., Long G. L. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus polymerase chain reaction. Anal Biochem. 1989 Jul;180(1):147–151. doi: 10.1016/0003-2697(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Nichol A. W., Angel L. A., Moon T., Clezy P. S. Lactoperoxidase haem, an iron-porphyrin thiol. Biochem J. 1987 Oct 1;247(1):147–150. doi: 10.1042/bj2470147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D. W., Neupert W. Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrias M. R., Findsen E. W., Gaul D. F., Knaff D. B. Resonance Raman characterization of a novel, oxygen-binding heme protein from Chromatium vinosum. Biochim Biophys Acta. 1984 Jul 17;788(1):87–97. doi: 10.1016/0167-4838(84)90300-5. [DOI] [PubMed] [Google Scholar]
- Osawa Y., Highet R. J., Bax A., Pohl L. R. Characterization by NMR of the heme-myoglobin adduct formed during the reductive metabolism of BrCCl3. Covalent bonding of the proximal histidine to the ring I vinyl group. J Biol Chem. 1991 Feb 15;266(5):3208–3214. [PubMed] [Google Scholar]
- Pettigrew G. W., Leaver J. L., Meyer T. E., Ryle A. P. Purification, properties and amino acid sequence of atypical cytochrome c from two protozoa, Euglena gracilis and Crithidia oncopelti. Biochem J. 1975 May;147(2):291–302. doi: 10.1042/bj1470291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler B. D., Erman J. E. Cytochrome c peroxidase compound I: formation of covalent protein crosslinks during the endogenous reduction of the active site. Biochim Biophys Acta. 1986 Jul 25;872(1-2):155–157. doi: 10.1016/0167-4838(86)90159-7. [DOI] [PubMed] [Google Scholar]


