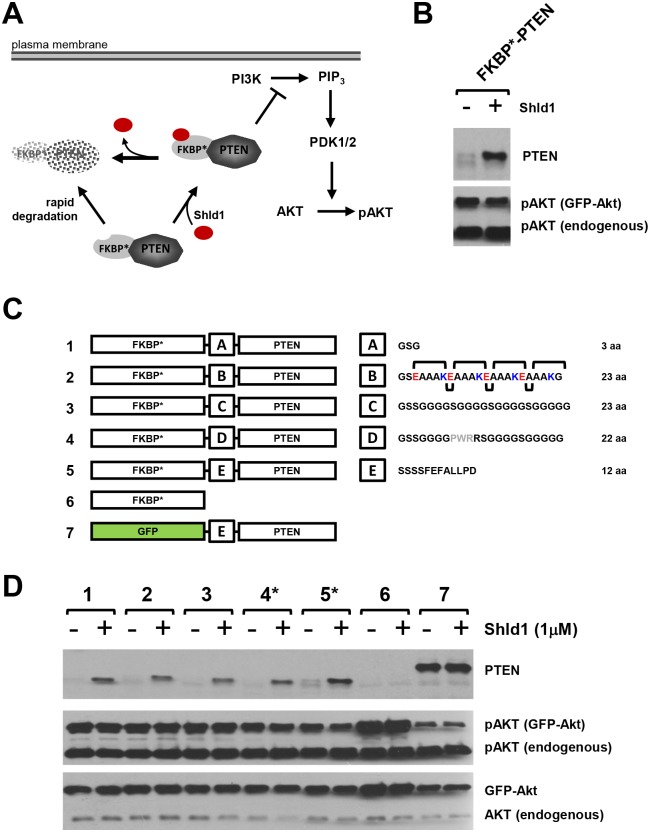
Fig 1. Consequence of linker optimization on PTEN activity of the FKBP*-PTEN fusion protein.
(A) Rationale of the DD-technology: Fusion of PTEN with a modified human FKBP variant harboring the F36V and L106P point mutations (FKBP*) leads to degradation of the fusion protein. Presence of the FKBP* ligand Shld1 confers stabilisation of FKBP*-PTEN and results in inhibition of PI3K/Akt signaling. (B) The fusion protein FKBP*-PTEN shows no PTEN activity. PTEN-deficient U87MG cells were transiently co-transfected with FKBP*-PTEN and AKT-GFP. After one day, cells were treated with Shld1 for 12 hours at 1μM before cell lysis and samples analyses by western blotting using indicated antibodies. (C) Consequence of linker optimization on PTEN activity of the FKBP*-PTEN fusion protein. Schematic representation of linker optimization. FKBP*-linker-PTEN variants with different linker regions (A-E) were generated. Linker A—short flexible linker; Linker B—a helix forming linker. Linker C—long flexible linker. Linker D—long flexible linker containing the PWR motif. Linker E—the original linker present in the enzymatic active GFP-PTEN. (D) Analyses of the effect of different linkers on PTEN activity in FKBP*-PTEN fusion variants in U87MG cells. Constructs expressing FKBP*-PTEN variants or GFP-PTEN were co-transfected with AKT-GFP into a PTEN null U87MG cells. Stabilization of FKBP*-PTEN or GFP-PTEN in presence or absence of Shld1 was monitored by PTEN antibody. The phosphorylation level of AKT-GFP at Ser473 served as a fast and effective experimental protocol to control for PTEN activity towards PIP3-dependent signaling in transfected cells, only. Only constructs 4 and 5, which contain proline in the linker sequence, showed moderate PTEN activity towards PI3K signaling.