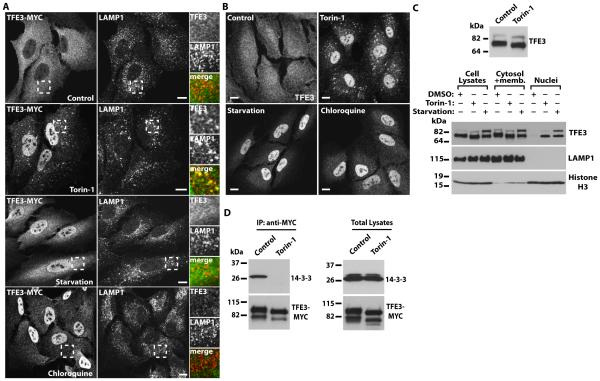
Fig. 1. TFE3 redistributes from the cytosol to the nucleus upon mTORC1 inactivation, nutrient deprivation, or lysosomal stress.
(A) Immunofluorescence confocal microscopy analysis of the subcellular distribution of TFE3-MYC in ARPE-19 cells incubated with Torin-1, starved in serum and amino acid-free medium, or exposed to Chloroquine for 1 hour. Cells were double stained with antibodies against TFE3 (a dilution of 1:7000 was used to detect recombinant TFE3) and LAMP1. Insets show a 2.5-fold magnification of the indicated region. Scale bars, 10 μm. Data are representative of 3 independent experiments and over 90% of cells exhibited the phenotypes shown. (B) Immunofluorescence confocal microscopy analysis of the subcellular distribution of endogenous TFE3 in ARPE-19 cells exposed to the same condition as those indicated in (A). Cells were stained with antibodies against TFE3 (a dilution of 1:200 was used to detect endogenous TFE3). Scale bars, 10 μm. Data are representative of 3 independent experiments and over 90% of cells exhibited the phenotypes shown. (C) Top: Immunoblotting showing changes on TFE3 electrophoretic mobility after 2 h incubation with Torin-1. Bottom: Immunoblotting analysis of the subcellular distribution of endogenous TFE3 in ARPE-19 cells incubated with DMSO or Torin-1 for 2 hours, or starved in a medium without serum and amino acids for 20 hours. The subcellular fractions were probed with antibodies against TFE3, LAMP1 (lysosomal membrane marker), and Histone H3 (nuclear marker). (D) Immunoblotting analysis of coimmunoprecipitated 14-3-3 with TFE3-MYC in ARPE-19 cells treated with DMSO (control) or Torin-1 for 1hour. Protein bands were detected with antibodies against MYC (used to detect TFE3-MYC) and 14-3-3. Data in C and D are representative of 3 independent experiments.