Abstract
Objectives
To determine whether lower extremity sensorimotor peripheral nerve deficits are associated with reduced walking endurance in older adults.
Design
Prospective cohort study with six years of follow-up.
Setting
Two U.S. clinical sites in (Pittsburgh, PA and Memphis, TN).
Participants
Community-dwelling older adults enrolled in Health, Aging and Body Composition study from the 2000/01 annual clinical examination (n=2393; age 76.5 ± 2.9 years; 48.2% male; 38.2% black) and subset with longitudinal data (n=1,178).
Interventions
Not applicable
Main Outcome Measures
Participants underwent peripheral nerve function examination in 2000/01, including peroneal motor nerve conduction amplitude and velocity, vibration perception threshold, and monofilament testing. Symptoms of lower-extremity peripheral neuropathy included numbness or tingling and sudden stabbing, burning, pain, or aches in the feet or legs. The long distance corridor walk (LDCW; 400m) was administered in 2000/01 and every two years afterwards for 6 years to assess endurance walking performance over time.
Results
In separate fully adjusted linear mixed models poor vibration threshold (>130 microns), 10-g and 1.4-g monofilament insensitivity were each associated with slower LDCW completion time (16.0, 14.1, and 6.7, seconds slower, respectively, P<.05 for each). Poor motor amplitude (<1mV), poor vibration perception threshold, and 10-g monofilament insensitivity were related to greater slowing/year (4.7, 4.3, and 4.3 additional seconds/year, respectively, P<.05), though poor motor amplitude was not associated with initial completion time.
Conclusions
Poorer sensorimotor peripheral nerve function is related to slower endurance walking and greater slowing longitudinally. Interventions to reduce the burden of sensorimotor peripheral nerve function impairments should be considered in order to help older adults to maintain walking endurance—a critical component for remaining independent in the community.
Keywords: peripheral nerve function, older adults, longitudinal analysis, walking endurance
Sensorimotor peripheral nerve function deficits are common in older adults, even in the absence of diabetes.1–3 In the Health, Aging, and Body Composition Study (Health ABC), recent work indicated that 55% of mobility-intact older adults (N=1,680; age 76.5 ± 2.9 years) at the 2000/01 examination had evidence of lower extremity peripheral nerve impairment.3 Poor peripheral nerve function in older adults is associated with worse lower extremity function,4–7 quadriceps and ankle dorsiflexion strength,8,9 falls,10–13 and lower extremity mobility limitations.3 Worse lower extremity sensation and motor control resulting from sensorimotor peripheral nerve dysfunction can lead to altered gait mechanics and inefficient and unstable gait patterns.14–19 Additionally, symptoms related to peripheral nerve impairments—including lower extremity pain or numbness—may make weight-bearing activities like walking difficult.
Walking endurance—the ability to walk for a sustained time or distance—is important for independence and remaining active in the community. Though aerobic fitness plays a major role in endurance walking, factors like peripheral nerve function may influence walking endurance over time, particularly given their impact on lower extremity function. However, work has been limited in exploring the impact of peripheral nerve impairments on walking endurance.
Evidence exists that walking endurance is worse in diabetic adults compared to non-diabetic healthy adults20 and also in the in the presence of a greater burden of lower extremity complications from diabetic peripheral neuropathy.21 In the InCHIANTI study, motor nerve conduction velocity was cross-sectionally associated with slower completion of a fast-paced 400m walking test for older diabetic and non-diabetic adults.22 Ideally, both motor and sensory nerve assessments and symptoms should be included to examine the full range of peripheral nerve function, not only clinical disease.23 Furthermore, no longitudinal studies have examined if peripheral nerve function contributes to decline in endurance walking over time in old age.
This study aimed to: 1) examine whether worse sensorimotor peripheral nerve function is cross-sectionally related to poorer endurance walking in older adults, and 2) determine whether worse sensorimotor peripheral nerve function is associated with greater slowing longitudinally over six years of follow-up in the Health ABC study.
METHODS
Study Population
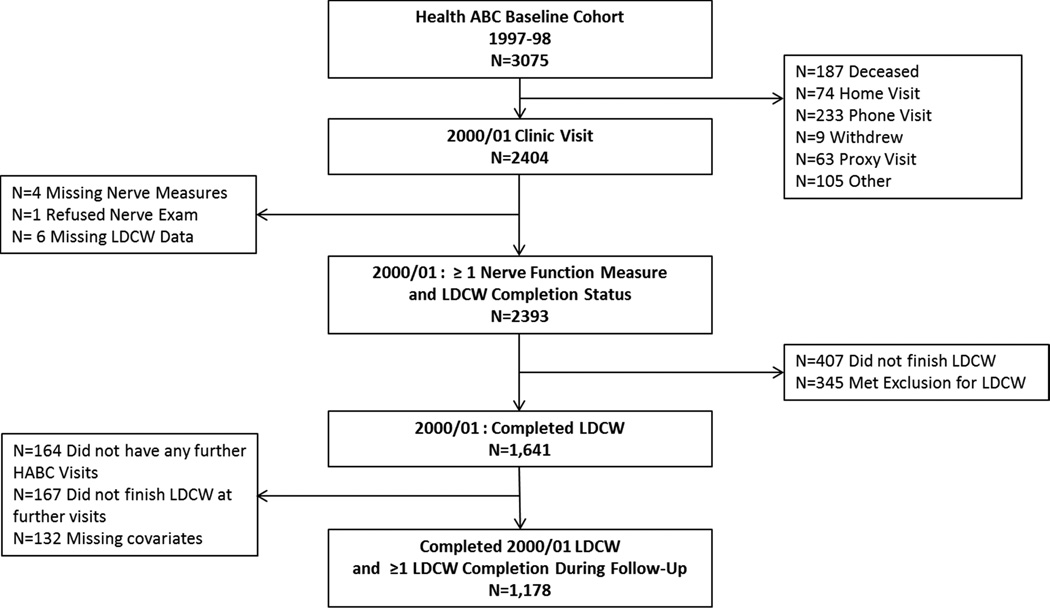
Participants were from Health ABC, a longitudinal cohort study of community-dwelling older adults (n=3075; age 70–79; 48.4% male; 41.6% black at baseline) from Pittsburgh, PA and Memphis, TN aimed at investigating factors related to the development of functional limitation and disability.24 Participants had to self-report having no difficulty in walking ¼ mile, climbing 10 steps, or any basic activity of daily living; be free of any life-threatening cancers; and plan to remain in the study area for at least three years. Participants completed the baseline visit between April 1997 and June 1998 and provided written informed consent. In 2000/01, 2,404 participants had a clinic visit, with 2393 having complete nerve function and endurance walking data (Figure 1). All study protocols were approved by institutional review boards at the University of Pittsburgh and University of Tennessee Health Science Center.
Figure 1.
Participant Flow Diagram from the Health, Aging and Body Composition Study
Peripheral Nerve Function Measures
Lower extremity sensory and motor nerve function was assessed in 2000/01 by a trained examiner. Motor nerve function was measured objectively using amplitude (millivolts) and conduction velocity (m/sec) of the peroneal motor response as previously described.25 Stimulation occurred at the popliteal fossa and ankle using the NeuroMax 8a. Sensory nerve function was measured using vibration detection threshold and monofilament testing. Vibration detection threshold (in microns) was measured at the bottom of the great toe with a VSA-3000 Vibratory Sensory Analyzerb. Monofilament insensitivity was defined as the inability to detect three of four touches at the dorsum of the large toe with a standard 10-g and light 1.4-g monofilamentc. Feet were warmed to 30°C, and measures were performed on the right unless contraindicated due to knee replacement, amputation, trauma, ulcer, or recent surgery, in which case the left side was tested unless also contraindicated. Clinically meaningful cut-points of motor amplitude <1 mV, conduction velocity <40 m/sec,26 or vibration threshold >130 were used to define impairment,. These cut-points were previously used by Ward and are related to quadriceps strength declines over time9 and incident mobility limitation.3
Self-reported symptoms of peripheral neuropathy included (1) numbness, "asleep feeling,” prickly feeling or tingling (2) sudden stabbing, burning, or deep aches, or (3) an open persistent sore or gangrene on either foot or leg in the past 12 months.
Endurance Walking
The long distance corridor walk (LDCW) was administered in 2000/01 and follow-up visits in 2002/03, 2004/05 and 2006/07 to assess walking endurance.27 Exclusion criteria included: systolic blood pressure >200 mmHg, resting pulse of ≥120 beats per minute, presence of an electrocardiogram abnormality, cardiac surgery, worsening of chest pain or shortness of breath in the prior three months. This test included minute warm-up where the participant was to “cover as much ground as possible” for two minutes followed by the LDCW performed “as quickly as possible at a pace that can be maintained for 400 meters.”28 Completion time for the 400m portion was recorded and used for analysis. Participants walked 10 laps around traffic cones placed 20m apart for a total of 400 meters. Heart rate was recorded for each lap and blood pressure was measured at the end of the test. The test was stopped if heart rate surpassed 135 beats per minute, or for lightheadedness, dizziness, chest pain, shortness of breath or leg pain.
Additional Covariates
Clinical site, age, sex, and race were included as demographic characteristics. Several factors were considered as potential covariates and were from the 2000/01 clinic visit unless otherwise noted.
Smoking history (assessed in 1999/2000), health status (excellent, very good, good, fair, or poor), current alcohol use (drinks/week), and physical activity (kilocalories expended/week in walking and stair climbing)29 were assessed via questionnaire. Body composition (fat mass and bone-free lean mass) was measured using dual-energy X-ray absorptiometry (DXA 4500A)d.
Diabetes was defined using self-reported physician’s diagnosis, hypoglycemic medication use, or fasting glucose ≥126 mg/dL (8 hour fast); impaired fasting glucose was defined as fasting glucose level of 100–125 mg/dL after an 8 hour fast.30 Arterial stiffening Peripheral arterial disease and arterial stiffening were defined ankle brachial index31 of <0.9 and >1.3, respectively. Hypertension was defined by self-report, medication use, measured systolic blood pressure ≥140mmHg or diastolic blood pressure ≥90mmHg. Poor vitamin B12 status was defined as <260 pmol/L32 and insufficient renal function as Cystatin-C >1mg/dL.33 Cognitive function was measured using the Digit Symbol Substitution Test34 at baseline and Modified Mini-Mental State Examination35 in 1999/2000. The Center for Epidemiologic Studies Depression Scale (CES-D) score assessed depressive symptoms.36 Prevalent coronary heart disease (bypass/coronary artery bypass graft, carotid endarterectomy, myocardial infarction, angina, or congestive heart failure), cerebrovascular disease (transient ischemic attack or stroke), lung disease (asthma, chronic obstructive pulmonary disease, or emphysema), and osteoarthritis in the knee or hip were assessed at baseline.
Statistical Methods
Participants were grouped based upon initial 2000/01 LDCW eligibility and completion. Groups included: ineligible for the LDCW, not completing the full LDCW, completing the 400m portion in >7 minutes, and completing in ≤7 minutes. The aerobic fitness validation study of the LDCW indicated that requiring >7 minutes is potentially indicative of significant functional limitations.27 Descriptive statistics were expressed using proportions for categorical variables and mean ± SD for continuous variables. Tests of trend were used to assess differences in characteristics and peripheral nerve function across groups. Linear mixed-effects models assessed the association between peripheral nerve function and greater slowing longitudinally over six years of follow-up. Only participants who completed the LDCW in 2000/01 and at least one follow-up could be included in the longitudinal models (n=1178, Figure 1). T-tests and chi-squared tests were used to compare characteristics of those who were included in the longitudinal models or not.
Some peripheral nerve function measures were moderately correlated and thus modeled separately. The date of each Health ABC visit was used as the time parameter. Interaction terms between each predictor and time indicate rate of change in 400m completion time over the course of the study contributed by that predictor. Time-varying covariates were used for factors that were updated throughout the study (physical activity, body composition). Models were built progressively using forward stepwise techniques, initially retaining factors reaching a significance of P<.10 or that appreciably attenuated the effect of peripheral nerve predictors. We started with unadjusted models including peripheral nerve function and the interaction between time and peripheral nerve function, and progressed by adding age, sex, race, site, and body composition for a minimally adjusted model. We further adjusted for baseline health status, lifestyle habits (smoking, alcohol consumption, and physical activity), prevalent diseases, and mental health and cognitive function variables as well as the time interactions for each of these covariates. Only factors reaching a significance of P<.05 were retained in the final parsimonious model. Because of the strong relationship between diabetes and peripheral nerve function, diabetes was retained in the final model regardless of significance. In sensitivity analyses, we tested the interaction between diabetes and peripheral nerve function, and we also reran the linear mixed models excluding participants with diabetes. No structure was imposed on the covariance matrix of the random effects. All models included a random intercept and a random slope for the visit parameter. Due to multiple comparisons, statistical significance was also checked after Bonferroni correction for eight comparisons.
RESULTS
Of the 2393 participants (age 76.5 ± 2.9 years; 48.2% male; 38.2% black), included in this analysis, n=345 (14.4%) were ineligible for the LDCW, n=407 (17.0%) started but did not complete the full LDCW, n=113 (4.7%) completed the 400m portion in >7 minutes, and 1528 participants (52.0%) completed in ≤7 minutes at the 2000/01 clinic visit. Significant trends existed that those who completed the LDCW were slightly younger, were less likely to be female or black, and were generally in better health and had fewer chronic conditions than those who were ineligible or unable to complete the full LDCW (Table 1).
Table 1.
Participant Characteristics by Long Distance Corridor Walk (LDCW) Eligibility and Completion in the Health, Aging and Body Composition Study 2000/01 Clinic Visit
| Participant Characteristics | Ineligible for LDCW n=345 |
Did Not Finish LDCW n=407 |
Completed LDCW (400m) in >7 Minutes n=113 |
Completed LDCW (400m) in ≤7 Minutes n=1528 |
P for Trend |
|---|---|---|---|---|---|
| Pittsburgh Site, N (5) | 192 (55.7) | 218 (53.6) | 49 (43.4) | 760 (49.7) | .03 |
| Age, Mean ± SD | 77.2 ± 2.9 | 76.8 ± 2.9 | 76.8 ± 3.0 | 76.3 ± 2.8 | <.001 |
| Female Sex | 197 (57.1) | 255 (62.7) | 71 (62.8) | 716 (46.9) | <.001 |
| Black Race | 192 (55.7) | 188 (46.2) | 61 (54.0) | 473 (31.0) | <.001 |
| Health Fair or Poor | 100 (29.2) | 92 (22.7) | 25 (22.3) | 127 (8.3) | <.001 |
| Body Composition | |||||
| Bone-Free Lean Mass (kg) | 48.6 ± 9.3 | 48.6 ± 9.7 | 47.4 ± 9.5 | 49.3 ± 10.7 | .24 |
| Fat Mass (kg) | 28.5 ± 10.1 | 28.6 ± 9.5 | 28.8 ± 10.4 | 24.9 ± 7.7 | <.001 |
| Lifestyle Habits | |||||
| Current Smoker | 34 (10.2) | 42 (10.4) | 13 (11.6) | 113 (7.5) | <.001 |
| Drink >1 Drink/Week | 84 (25.2) | 95 (23.6) | 24 (21.4) | 509 (33.6) | <.001 |
| Physical Activity* (kcal/kg/week) | 3.1 ± 6.7 | 3.6 ± 6.0 | 3.0 ± 7.4 | 7.3 ± 20.1 | <.001 |
| Prevalent Diseases and Conditions | |||||
| Diabetes | 103 (29.9) | 117 (28.8) | 34 (30.1) | 263 (17.2) | <.001 |
| Impaired Fasting Glucose | 54 (15.7) | 73 (18.0) | 15 (13.3) | 242 (15. | |
| Hypertension | 261 (77.9) | 272 (67.5) | 77 (68.8) | 822 (54.5) | <.001 |
| Coronary Heart Disease | 73 (21.5) | 105 (26.3) | 17 (15.2) | 222 (14.7) | <.001 |
| Cerebrovascular Disease | 38 (11.5) | 39 (9.9) | 8 (7.3) | 72 (4.8) | <.001 |
| Lung Disease | 85 (24.6) | 105 (26.3) | 20 (17.7) | 229 (15.0) | <.001 |
| Osteoarthritis (Knee or Hip) | 59 (17.1) | 57 (14.0) | 16 (14.2) | 138 (9.0) | <.001 |
| Peripheral Arterial Disease | 90 (28.8) | 94 (24.5) | 21 (19.4) | 167 (11.2) | <.001 |
| Arterial Stiffening | 14 (4.5) | 17 (4.4) | 9 (8.3) | 79 (5.2) | <.001 |
| Insufficient Renal Function (Cystatin-C >1mg/dL) | 139 (41.1) | 143 (35.9) | 41 (37.6) | 335 (22.4) | <.001 |
| Poor Vitamin B12 (<260 pmol/L) | 52 (16.0) | 65 (16.7) | 18 (17.1) | 344 (17.4) | .51 |
| Shortness of Breath While Walking | 154 (44.6) | 178 (43.7) | 43 (38.1) | 344 (22.5) | <.001 |
| Mental Health and Cognitive Function | |||||
| CES-D Score | 8.2 ± 7.7 | 7.4 ± 7.0 | 7.6 ± 6.0 | 5.7 ± 5.8 | <.001 |
| 3MSE Score | 87.9 ± 8.9 | 89.0 ± 8.8 | 86.9 ± 9.6 | 91.6 ± 7.3 | <.001 |
| Digit Symbol Substitution Score | 31.7 ± 14.4 | 34.7 ± 14.5 | 31.6 ± 15.2 | 28.6 ± 14.2 | <.001 |
Cross-sectional LDCW eligibility and performance were also associated with higher motor amplitude, lower vibration threshold, detection of both monofilaments, and reporting no symptoms of peripheral neuropathy (Table 2, P<.05 for each). Motor nerve conduction velocity was not associated with LDCW eligibility or completion.
Table 2.
Year 4 Peripheral Nerve Function by LDCW Eligibility/Completion Group in the Health, Aging and Body Composition study 2000/01 Clinic Visit
| Peripheral Nerve Function Measure |
Ineligible for LDCW n=345 |
Did Not Finish LDCW n=407 |
Completed LDCW (400m) in >7 Minutes n=113 |
Completed LDCW (400m) in ≤7 minutes n=1528 |
P for trend |
|---|---|---|---|---|---|
| Motor Nerve Function | |||||
| Motor amplitude, mV, Mean ± SD | 3.0 ± 2.1 | 3.1 ± 2.0 | 3.1 ± 2.0 | 3.5 ± 2.0 | <.001 |
| Poor amplitude, N (%) | 47 (19.9) | 41 (13.8) | 12 (15.0) | 110 (8.8) | <.001 |
| Sensory Nerve Function | |||||
| Conduction velocity, m/sec | 43.2 ± 5.6 | 43.3 ± 5.2 | 44.8 ± 5.7 | 43.7 ± 5.4 | .33 |
| Poor conduction velocity | 52 (21.1) | 63 (22.7) | 15 (20.3) | 262 (21.9) | .37 |
| Vibration threshold, microns | 56.0 ± 39.5 | 52.5 ± 35.4 | 55.2 ± 35.8 | 50.1 ± 34.7 | .03 |
| Poor vibration threshold | 32 (9.9) | 19 (4.8) | 10 (9.2) | 74 (5.0) | .008 |
| 1.4 monofilament insensitivity | 127 (38.3) | 35 (36.7) | 84 (42.7) | 23 (36.2) | <.001 |
| 10-g monofilament insensitivity | 43 (13.0) | 41 (10.3) | 16 (14.6) | 107 (7.1) | |
| Symptoms of Peripheral Neuropathy | |||||
| One symptom | 105 (31.0) | 125 (31.0) | 34 (30.1) | 379 (24.9) | <.001 |
| Two or More Symptoms | 65 (19.2) | 46 (11.4) | 7 (6.2) | 120 (7.9) |
Compared to those included in the longitudinal analyses, those not included were slightly older (mean age 76.9 ± 2.9 vs. 76.2 ± 2.8 years, P<.001), more likely to be women (55.8% vs. 52.1%, P<.001) and more likely to be black (44.9% vs. 32.2%, P<.001). Those not in the longitudinal analyses also had worse motor nerve amplitude (3.1 ± 1.9 vs. 3.6 ± 2.0 mV, P<.001), higher vibration perception threshold (53.6 ± 36.4 vs. 49.5 ± 34.6 microns, p=0.005), were more likely to have 10-g (10.7% vs. 7.0%) or 1.4-g (37.6% vs. 36.1%) monofilament insensitivity (p=0.004), and were more likely to report at least one symptom of peripheral neuropathy (42.3% vs. 31.8%). The most common reasons for not being included in the mixed models were not finishing the initial LDCW (n=407) or meeting exclusion criteria at the initial LDCW assessment (n=345).
In longitudinal analyses, worse motor amplitude worse vibration threshold and poor vibration threshold, 10-g and 1.4-g monofilament insensitivity were associated with 3.9, 5.2, 19.6, 9.3, and 20.7 seconds slower initial 400m walk completion time, respectively, when adjusting for age, sex, race, site, height, and weight (Table 3; P<.05 for all). Relationships were slightly attenuated after further adjusting for health habits and health conditions. Significant relationships remained with the exception of lower motor amplitude (per SD poorer amplitude). Poor vibration perception threshold was associated with completing the 400m walk 16.0 seconds slower and an additional 4.3 seconds of slowing per year compared to those with vibration perception threshold ≤130 microns. Those with 10-g monofilament insensitivity completed the 400m walk 14.1 seconds slower and slowed an additional 4.3 seconds each year. Longitudinal endurance walking was not associated with either motor nerve conduction velocity or symptoms of peripheral neuropathy. In sensitivity analyses, no significant interaction existed between diabetes and peripheral nerve function. All results remained consistent after excluding individuals with diabetes.
Table 3.
Peripheral nerve functioning and longitudinal performance in LDCW from 2000/01 to 2006/07 in the Health, Aging and Body Composition study
| Model 1 | Model 2 | Model 3: Final Model |
||||
|---|---|---|---|---|---|---|
| Standardized Betas | Standardized Betas | Standardized Betas | ||||
| Main Effect |
Time Interaction |
Main Effect |
Time Interaction |
Main Effect |
Time Interaction |
|
| Motor Nerve Function | ||||||
| SD lower amplitude, mV | 3.9* | 0.5 | 3.7* | 0.3 | 2.2 | 0.2 |
| Poor amplitude | 5.8 | 4.8** | 3.2 | 4.4** | −0.8 | 4.7**† |
| SD Slower Conduction Velocity, m/sec | −0.8 | −0.1 | −0.5 | 0.3 | 1.1 | 0.3 |
| Poor Conduction Velocity | −2.1 | −0.04 | 0.9 | −0.3 | −3.9 | −0.7 |
| Sensory Nerve Function | ||||||
| SD higher vibration perception threshold, microns | 5.2** | 1.6** | 4.9** | 1.7** | 3.4* | 1.5**† |
| Poor vibration perception threshold | 19.6** | 4.1* | 20.1** | 4.9** | 16.0* | 4.2* |
| 1.4 monofilament insensitivity | 9.3** | −0.6 | 8.6** | −0.6 | 6.9* | −0.9 |
| 10-g monofilament insensitivity | 20.7** | 4.4** | 16.3** | 4.5** | 14.4** | 3.8**† |
P<.05,
P≤.01.
P<.05,
P≤.01,
Significant using Bonferroni adjustment for 8 multiple comparisons, P<0.006).
Note: Model 1 is adjusted for age, sex, race and site; Model 2 Adjusted for Model 1 + health status, physical activity, current cigarette smoking, lean mass and fat mass; Model 3 Motor amplitude and conduction velocity: Model 2 + PAD, cerebrovascular disease, diabetes, insufficient renal function, shortness of breath while walking, and DSST score. Cigarette smoking and health status were removed. Model 3: Sensory nerves function: Model 2 + PAD, cerebrovascular disease, diabetes, insufficient renal function, DSST score, CES-D and hypertension. Cigarette smoking and health status were removed. Model 3: Vibration perception threshold: Model 2 + PAD, cerebrovascular disease, diabetes, insufficient renal function, DSST score, CES-D and hypertension, health status removed due to non-significance. Beta values for continuous predictors represent the decrease/increase in LDCW completion time based upon each standard deviation change representing worse nerve functioning. Continuous nerve function mean ± SD of those in the longitudinal sample (n=1178): Amplitude = 3.6 ± 2.0 mV, conduction velocity = 43.7 ± 5.4, and vibration threshold = 49.5 ± 34.6. Time interactions indicate the additional increase in LDCW completion time per year (change in slope) compared to the baseline category (for categorical variables) or for each standard deviation of worse nerve functioning.
DISCUSSION
In this cohort of community-dwelling older adults, worse motor and sensory peripheral nerve function was related to worse endurance walking performance, and sensory nerve impairments were associated with greater slowing longitudinally. Additionally, peripheral nerve function and presence of symptoms of neuropathy were cross-sectionally related to the inability to complete the initial LDCW. Though peripheral nerve impairments are often thought to be primarily a concern for those with diabetes, these results support previous work indicating that peripheral nerve function is an important predictor of mobility related outcomes in older adults independent of diabetes.3,4
Vibration perception threshold and standard monofilament insensitivity were strongly associated with the slowing of endurance walking over time. Sensory nerves detect touch, vibration, and other sensations regarding the external environment, while motor nerves relay signals from the central nervous system that allow for voluntary movement. Sensory nerve function in the lower extremities is crucial for perception of joint position, posture, and balance—all factors that play roles in walking quickly and efficiently. Worse vibration perception threshold is associated with worse balance and slower usual gait speed in older adults without diabetes or overt peripheral neuropathy.4,7,37
Little work exists relating sensorimotor peripheral nerve function to endurance walking in older adults. In InCHIANTI, slower motor nerve conduction velocity was associated with slower completion of a fast-paced 400m walking test for older adults,22 though this study did not include measures of motor nerve amplitude, sensory peripheral nerve function or the assessment of symptoms of peripheral neuropathy. The association with conduction velocity is in contrast to our results, though age and racial differences between the InCHIANTI and Health ABC cohorts may have contributed to the differences in findings. Worse motor nerve amplitude (a sign of axonal degeneration) but not worse motor nerve conduction velocity (a sign of demyelination) have been shown to be associated with worse lower extremity physical performance,4 slower usual gait speed4, and incident lower extremity limitation3 in Health ABC.
The effect size of poor sensory peripheral nerve function was similar in magnitude to those of common risk factors associated with worse walking endurance. For example, current smoking was independently associated with completing the initial 400m walk 14.0 seconds slower, and cerebrovascular disease was associated with 16.1 seconds slower on the initial 400m walk, though neither were significantly associated with greater slowing over time. This is similar to the effect of poor vibration perception threshold (16.0 seconds slower) and 10-g monofilament insensitivity (14.1 seconds) on initial 400m walk completion time, which were each related to greater slowing over time (4.3 seconds slower each).
Peripheral nerve impairments are common in older adults,1 and improving functional outcomes in those with these impairments may be important on many levels. Work in diabetic populations has shown that worse lower extremity peripheral nerve function is associated with altered gait biomechanics17–19,38 Because of the associations between gait alterations with injuries and falls16,38–41 these factors may also be in the pathway to worse lower-extremity function for older adults. Physical activity may help older adults delay persistent mobility limitations,42 however, whether physical activity interventions may be used to reduce physical functional impairments due to poor peripheral nerve function is currently unknown.
A major strength of this work is the inclusion of several measures of both motor and sensory nerve function, as well as symptoms associated with peripheral neuropathy. Nerve function deficits in older adults are commonly asymptomatic,1 making it particularly important to include a variety objective and subjective measures. In addition, the large sample size and longitudinal design allowed an examination of several factors that could potentially influence peripheral nerve function and/or walking endurance over time.
Study Limitations
Only participants who completed the initial LDCW and at least one follow-up could be used in the longitudinal analysis. Those who could not complete initial LDCW or at least one follow-up had worse peripheral nerve function compared to those who were included in the longitudinal analyses. Retention bias is applicable to most cohort studies of older adults since those who return for clinic visits are typically healthier than those who do not.43 Thus, we may have underestimated the true effect of peripheral nerve function on the worsening of walking endurance. Finally, though the LDCW is a valid measure for estimating aerobic fitness in older adults,27 traditional measures of maximal aerobic capacity were not available in this study. The relationship between aerobic fitness as determined from a maximal treadmill test and also other physical activity assessments to peripheral nerve function has should be explored in future work. Our physical activity assessment was restricted walking and stair climbing, as these were the only activities self-reported consistently throughout this follow-up period.
CONCLUSIONS
Poorer sensory and motor peripheral nerve function in older adults is related to slower endurance walking and greater slowing longitudinally over 6 years. Declines in endurance walking likely contribute to the development of mobility limitations for those with sensorimotor peripheral nerve impairments. Future work to reduce the burden of these impairments should be considered to help older adults to maintain walking endurance—a crucial component for community independence.
ACKNOWLEDGMENTS
This research was supported by National Institute on Aging Contracts N01-AG-6–2101, N01-AG-6–2103, and N01-AG-6–2106 and Grant 1-R01-AG 028050 (to ESS) and National Institute of Nursing Research Grant R01-NR012459 and supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, the University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30-AG024827) Pilot Grant (to ESS).
List of Abbreviations
- Health ABC
Health, Aging, and Body Composition Study
- LDCW
Long Distance Corridor Walk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented to the Gerontological Society of America, November 6, 2014, Washington, D.C.
Disclosure: Dr. Vinik reports a financial relationship with Merck, ISIS Pharmaceuticals, and PamLab outside the submitted work. The other authors have nothing to disclose.
SUPPLIERS
XLTEK, 2568 Bristol Cir, Oakville, ON L6H 5S1, Canada
Medoc Advanced Medical Systems U.S. 1502 W Nc Highway 54 Ste 404, Durham, NC 27707
North Coast Medical, 8100 Camino Arroyo, Gilroy, CA 95020.
Hologic, Inc., 35 Crosby Dr. Bedford, MA 01730
References
- 1.Gregg EW, Sorlie P, Pulose-Ram R, Eberhardt M, Wolz M, Burt V, et al. Prevalence of lower-extremity disease in the U.S. adult population 40+ years of age with and without diabetes. Diabetes Care. 2004;27(27):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 2.Baldereschi M, Inzitari M, Di Carlo A, Farchi G, Scafato E, Inzitari D. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007;68(18):1460–1467. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- 3.Ward RE, Boudreau RM, Caserotti P, Harris TB, Zivkovic S, Goodpaster BH, et al. Sensory and motor peripheral nerve function and incident mobility disability. J Am Geriatr Soc. 2014;62(12):2273–2279. doi: 10.1111/jgs.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strotmeyer ES, De Rekeneire N, Schwartz A, Faulkner K, Resnick H, Goodpaster B, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults. Diabetes Care. 2008;31(9):17671–11772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cimbiz A, Cakir O. Evaluation of balance and physical fitness in diabetic neuropathic patients. J Diabetes Complicat. 2005;19(3):160–164. doi: 10.1016/j.jdiacomp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Resnick HE, Stansberry K, Harris T, Tirivedi M, Smith K, Morgan P, et al. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25(1):43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 7.Resnick H, Vinik A, Schwartz AV, Leveille S, Brancati F, Balfour J, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women's Health and Aging Study. Diabetes Care. 2000;23(11):1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 8.Strotmeyer ES, de Rekeneire N, Schwartz AV, Resnick HE, Goodpaster B, Faulkner K, et al. Sensory and Motor Peripheral Nerve Function and Lower-Extremity Quadriceps Strength: The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57(11):2004–2010. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward RE, Boudreau RM, Caserotti P, Harris TB, Zivkovic S, Goodpaster BH, et al. Sensory and motor peripheral nerve function and longitudinal changes in quadriceps strength. J Gerontol A Biol Sci Med Sci. 2015 Apr;70(4):464–470. doi: 10.1093/gerona/glu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson J, Ching C, Hurvitz E. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992;40(10):1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz A, Vittinghoff E, Sellmeyer D, Feingold K, de Rekeneire N, Strotmeyer E, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31(3):391–396. doi: 10.2337/dc07-1152. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrucci L, Bandinelli S, Cavazzini C, Lauretani F, Corsi A, Bartali B, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 116(12):807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Lalli P, Chan A, Garven A, Midha N, Chan C, Brady S, et al. Increased gait variability in diabetes mellitus patients with neuropathic pain. J Diabetes Complicat. 2013;27(3):248–254. doi: 10.1016/j.jdiacomp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Mueller M, Minor S, Sahrmann S, Schaaf J, Strube M. Differences in the Gait characteristics of patients with diabetes and peripheral neuropathy compared with agematched controls. Physical Therapy. 1994;74(4):299–308. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- 15.Katoulis E, Ebdo-Parry M, Lanshammar H, Vileikyte L, Krlkarni J, Boulton A. Gait abnormalities in diabetic neuropathy. Diabetes Care. 1997;20(12):1904–1907. doi: 10.2337/diacare.20.12.1904. [DOI] [PubMed] [Google Scholar]
- 16.Raspovic A. Gait Characteristics of people with diabetes-related peripheral neuropathy, with and without a history of ulceration. Gait Posture. 2013;38:723–728. doi: 10.1016/j.gaitpost.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Brach J, Talkowiski J, Strotmeyer E, Newman A. Diabetes mellitus and gait dysfunction: possible explanitory factors. Physical Therapy. 2008;88(11):1365–1374. doi: 10.2522/ptj.20080016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschamps K, Matricali GA, Roosen P, Nobels F, Tits J, Desloovere K, et al. Comparison of foot segmental mobility and coupling during gait between patients with diabetes mellitus with and without neuropathy and adults without diabetes. Clin Biomech. 2013;28(7):813–819. doi: 10.1016/j.clinbiomech.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Menz H, Lord S, St George R, Fitzpatrick R. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehab. 2004;85(2):245–252. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Özdirenç M, Biberoğlu S, Özcan A. Evaluation of physical fitness in patients with Type 2 diabetes mellitus. Diabetes Res Clin Pr. 2003;60(3):171–176. doi: 10.1016/s0168-8227(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 21.Kanade RV, Deursen RWM, Harding K, Price P. Walking performance in people with diabetic neuropathy: benefits and threats. Diabetologia. 2006;49(8):1747–1754. doi: 10.1007/s00125-006-0309-1. [DOI] [PubMed] [Google Scholar]
- 22.McDermott M, Guralnik J, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: The InCHIANTI Study. J Am Geriatr Soc. 2004;52(3):405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 23.Tesfaye S, Boulton A, Dyck P, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonsick E, Newman A, Nevitt M, Kkritchevsky S, Ferrucci L, Guralnik J, et al. Measuring higher level physical function in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2001:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 25.Ward RE, Boudreau RM, Vinik AI, Zivkovic S, Njajou O, Satterfield S, et al. Reproducibility of peroneal motor nerve conduction measurement in older adults. Clin Neurophysiol. 2013;124(3):603–609. doi: 10.1016/j.clinph.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maser RE, Nielsen VK, Dorman JS, Drash AL, Becker DJ, Orchard TJ. Measuring subclinical neuropathy: does it relate to clinical neuropathy? Pittsburgh epidemiology of diabetes complications study-V. J Diabet Complications. 1991;5(1):6–12. doi: 10.1016/0891-6632(91)90003-8. [DOI] [PubMed] [Google Scholar]
- 27.Simonsick E, Fan E, Fleg J. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2005:127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Simonsick E, Montgomery P, Newman A, Bauer D, Harris T. Measuring fitness in healthy older adults: the Health ABC long distance corridor walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth B, Haskell W, Leon A. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993:71–83. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboyans V, Criqui M, Abraham P, Allison M, Creager M, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 32.Leishear K, Boudreau RM, Studenski SA, Ferrucci L, Rosano C, de Rekeneire N, et al. Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. J Am Geriatr Soc. 2012;60(6):1057–1063. doi: 10.1111/j.1532-5415.2012.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shlipak M, Wassel Fyr C, Chertow G, Harris T, Kritchevsky S, Tylavsky F, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17(1):254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 34.Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 35.Teng E, Chui H. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 36.Radloff L. The CES-D Scale: A self-report depression xcale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 37.Buchman AS, Wilson RS, Leurgans S, Bennett DA. Vibratory thresholds and mobility in older persons. Muscle Nerve. 2009;39(6):754–760. doi: 10.1002/mus.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zurales K, DeMott TK, Kim H, Allet L, Ashton-Miller JA, Richardson JK. Am J Phys Med Rehabil. 2015 Jun 5; doi: 10.1097/PHM.0000000000000324. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tinetti M, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 40.Hausdorff JM, Edelberg HK, Mitchell S, Goldberger A, Wei J. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehab. 1997 Mar;78:278–286. doi: 10.1016/s0003-9993(97)90034-4. (1997) [DOI] [PubMed] [Google Scholar]
- 41.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehab. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 42.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The life study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strotmeyer E, Arnold A, Boudreau R, Ives D, Cushman M, Robbins J, et al. Long-term retention of older adults in the Cardiovascular Health Study: implications for studies of the oldest old. J Am Geriatr Soc. 2010 Apr;58(4):696–701. doi: 10.1111/j.1532-5415.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]