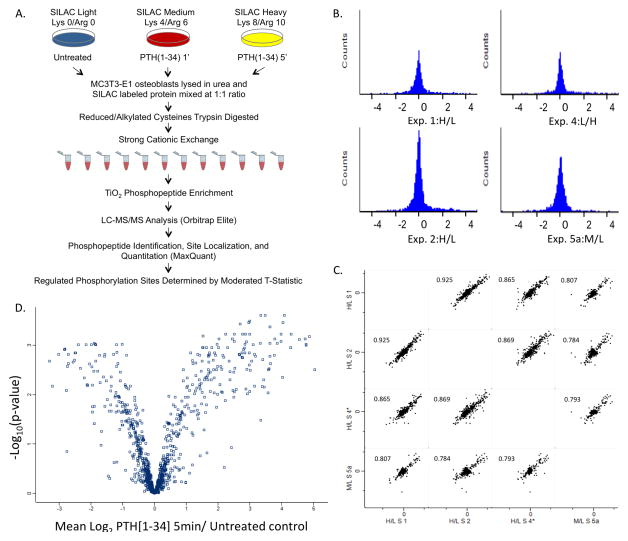
Figure 1.
Capturing the PTH1R regulated phosphoproteome. A) Experimental workflow for measuring differential changes in PTH(1-34)-induced phosphorylation events following acute stimulation of the receptor. SILAC labeled cells were differentiated for 10 days in light, medium, or heavy SILAC osteogenic media, serum starved in 1% FCS overnight and stimulated with hPTH[1-34] for varying times. Cells were lysed and the protein was mixed 1:1. The mixture was trypsin digested and the resulting peptides fractionated by strong-cation exchange (SCX). Phosphopeptides were enriched from each fraction with TiO2 and analyzed by LC-MS/MS (Orbitrap Elite, Thermo Scientific). Peptide identification and relative changes in the extent of phosphorylation were determined using MaxQuant. Statistical analysis to determine significantly regulated sites was performed using a moderated t-test. B) The distribution of normalized, log2 transformed ratios of phosphopeptides enriched from hPTH(1-34) MC3T3-E1 cells treated for 5 minutes compared to control cells. The histograms of SILAC encoded peptide ratios measured in four biological replicate experiments including a label swap control exhibited a normal distribution. C) Multi-scatter plots representing pairwise comparisons of the normalized log2 ratios measured in each experiment demonstrate high Pearson correlation coefficients (>0.75) indicating reproducibility of measurements between biological replicate experiments. D) Volcano plot showing the reproducibility of measured ratios for each phosphopeptide that was observed at least twice.