Abstract
Kisspeptin, a regulator of reproductive function and puberty in mammals, is expressed in the rostral periventricular nucleus (AVPV) and arcuate nucleus (Arc) and its expression is at least partially regulated by estradiol in rodents. The aim of the present study was to determine contributions of genetic factors and gonadal steroid hormones in the sexual differentiation of kisspeptin immunoreactive cell populations in the AVPV and Arc during postnatal development using agonadal Steroidogenic factor-1 knockout (SF-1 KO) mice. To examine effects of gonadal hormones on pubertal development of kisspeptin neurons, SF-1 KO mice were treated with estradiol benzoate (EB) from P25 to P36 and their brains examined at P36. No sex differences were observed in SF-1 KO mice during postnatal development and after treatment with EB, which failed to increase the number of kisspeptin-ir cells at P36 in SF-1 KO mice to the levels found in WT control females. This suggests that specific time periods of estradiol actions or other factors are needed for sexual differentiation of the pattern of immunoreactive kisspeptin in the AVPV. Kisspeptin immunoreactivity in the Arc was significantly higher in gonadally intact WT and SF-1 KO females than male mice at P36 during puberty. Further, in WT and SF-1 KO females, but not in males, adult levels were reached at P36. This suggests that maturation of the kisspeptin system in the Arc differs between sexes and is regulated by gonad- independent mechanisms.
Keywords: kisspeptin, sex difference, brain, estradiol, genetic factor, steroidogenic factor 1, SF-1 KO mouse
Introduction
Kisspeptin (Kiss1) and its receptor, G protein- coupled receptor 54 (GPR54, Kiss1r), have been implicated in reproduction and sexual maturation as key regulators of gonadotropin releasing hormone (GnRH) secretion in mammals including humans [1-10]. The neuroanatomy of Kiss1 expressing neurons differs among mammalian species [11]. In the rodent brain two populations of Kiss1 expressing neurons reside in the rostral periventricular nucleus (AVPV) and in the arcuate nucleus of the hypothalamus (Arc) [12-15]. Regulation of Kiss1 expression differs between these neural populations. In the AVPV, neurons expressing kisspeptin can be detected by immunohistochemistry (IHC) on postnatal day 10 (P10) in male and female mice [16]. Afterwards, the number of kisspeptin immunoreactive (kisspeptin-ir) neurons progressively increases in a sex specific manner until the onset of puberty, so that mature female mice have approximately ten times more kisspeptin-ir neurons than males [16]. Several lines of evidence suggest the involvement of steroid hormones in the sexual differentiation of kisspeptin neurons. Co-localization studies showing Kiss1 expressing neurons in the AVPV co-express all major receptors for steroid hormones (estrogen receptor α (ERα) and β (ERβ), androgen and progesterone receptors) [12,17,18]. Studies in rodents have shown that sex specific development of Kiss1 neurons in the AVPV depends on both organizational and activational effects of gonadal steroid hormones. Treatment with androgens during the first postnatal week masculinized the number of Kiss1 mRNA expressing neurons in adult female rats [15,19] and neonatal castration of male rats blocked masculinization of the number of kisspeptin-ir neurons [19], suggesting that a male phenotype is a consequence of permanent organizing actions of gonadal hormones on developing Kiss1 neurons in the AVPV. Development of the full female complement of kisspeptin-ir neurons in the AVPV in gonadectomized WT mice depends on the exposure to estrogens during puberty from P22 to P30 [20] although a study by Kim et al. [21] suggested that at the level of mRNA (but not peptide) expression, feminization of Kiss1 expressing neurons might start earlier. In adulthood, Kiss1 mRNA and kisspeptin expression in the AVPV depends on activational effects of gonadal steroids as mRNA (in situ hybridization; ISH) and peptide (IHC) were decreased after gonadectomy and restored by estradiol replacement [12,13,18,20]. The effects of gonadal steroid hormones have been reinforced by studies with ERα (ERKO) and aromatase (ARKO) knockout mice [12,13,22,23].
Kiss1 mRNA and kisspeptin immunoreactivity in the Arc can be detected during early fetal development in mice and rats [24,25] and persists throughout prenatal and postnatal development [25-27]. Several sex differences in Kiss1 mRNA and kisspeptin expression in the Arc in developing brain have been reported in gonadally intact rodents. During embryonic development and in adulthood sex differences in the mRNA content from dissected hypothalami and in the number of Kiss1 mRNA containing cells have been reported in mice [27]. Kiss1 mRNA levels during neonatal, prepubertal and pubertal development [28] and in adulthood [14] have been reported to be higher in female than male rats. Similarly, sex differences have been reported also at the peptide level from neonatal period to adulthood in rats [26] and during early postnatal development from P10- P25 in mice [23]. Previous studies have shown that gonadal steroid hormones regulate Kiss1 mRNA levels and kisspeptin immunoreactivity in the Arc. Kiss1 mRNA levels increased after gonadectomy and decreased by E2, T and DHT replacement in mice and rats [12-15,19]. However, at the peptide level, decreased levels of immunoreactive kisspeptin following gonadectomy were restored with E2 or DHT treatment in adult mice [23]. The requirement for estradiol to induce kisspeptin immunoreactivity in the Arc has also been suggested by studies in mice with ERα ablated Kiss1 neurons (KERKO, [22]) and ARKO mice [23], which both had diminished kisspeptin immunoreactivity during postnatal development. Similarly as for the AVPV kisspeptin neurons, neonatal exposure to estradiol benzoate (EB) significantly decreased kisspeptin immunoreactivity in the Arc in female rats [29].
Early studies demonstrated that central mechanisms regulating puberty onset are not gonad dependent [30,31]. These observations have been confirmed by recent studies showing profound changes in Kiss1 mRNA levels and kisspeptin immunoreactivity under constant low peripheral estrogenic environments [26] or low levels of gonadal steroid hormones [32] and by RT- PCR, ISH and IHC studies showing that Kiss1 expression in the Arc might be regulated by other factors such as members of Polycomb (PcG) protein family [33], neuropeptides [34-41], trophic factors [42], and neurotransmitters [43,44]. Despite the progress toward identification of the gonad- independent factors involved in the regulation of Kiss1 expression in the Arc during development in recent years, their role in the sexual differentiation of Kiss1 expression in the Arc requires further clarification.
Gonadal steroid hormones and genes on sex chromosomes are two major factors influencing brain sexual differentiation in mammals [45-47]. Two genetic mouse models have provided a better understanding of contributions of sex chromosomes to the brain sexual differentiation and function; namely the four core genotype (FCG) model [48-51] and mice with the disruption of the steroidogenic factor-1 gene (Nr5a1) (SF-1 KO) [52,53]. In SF-1 KO mice, genital ridges disintegrate early during embryonic development [54], before the initiation of steroidogenesis in fetal testes [55]. Therefore, these mice are not exposed to endogenous gonadal steroid hormones and when compared to hormonally manipulated WT control mice they represent a unique model for studying genetic and hormonal influences to brain sexual development separately [47].
The aim of the present study was to determine whether there are any sex differences in the production of immunoreactive kisspeptin in AVPV and Arc after birth in agonadal SF-1 KO mice either without hormones and after treatment with estradiol. An additional goal was to determine if a peripubertal window of estradiol exposure from P25 to P36 for development of kisspeptin immunoreactivity is sufficient to induce normal levels of immunoreactive kisspeptin in agonadal SF-1 KO mice in the AVPV area.
Materials and methods
Animals and brain recovery
Heterozygous mice with a disrupted sf-1 (Nr5a1) allele (SF1+/-) were backcrossed for more than 10 generations to C57BL/6J mice to produce a congenic line. All mice were housed under standard laboratory conditions at the University of Ljubljana Veterinary faculty in a 12:12 light/dark cycle (lights off at 1800 h) with phytoestrogen free diet (#2916, Harlan Teklad, Milano, Italy) and water ad libitum. All animal experiments were conducted according to the ethical principles and in accordance with EU directive (2010/63/EU). Animal experiments were approved by the Veterinary commission of Slovenia and the Animal Care and Use Committee at Colorado State University.
SF-1 +/- mice were mated to produce homozygous SF-1 knockout (SF-1 KO) and control wild type (WT) offspring. Due to the lack of gonads during fetal development, SF-1 KO mice develop female reproductive organs and external genitalia regardless of genetic sex. In the present study SF-1 KO mice were sexed according to their chromosomal sex, i.e. XY= SF-1 KO males and XX= SF-1 KO females. To ensure survival of SF-1 KO mice, all newborn pups were injected daily for 6-7 days with 50 μl of a corticosteroid cocktail in a corn oil (Sigma) (sc 400 μg/ml hydrocortisone, 400 ng/ml dexamethasone, and 500 ng/ml fludrocortisone acetate; all from Sigma, Steinheim, Germany). Mice were genotyped by PCR assay of tail DNA on day 6 or 7 after birth as previously described [56]. Female WT littermates or female pups from other C57BL/6J litters born within 3 days were used as a source of adrenal transplants; these techniques have been reported previously [57]. After adrenal transplantation, SF-1 KO mice received three more corticosteroid injections on days 9, 12 and 16 until weaning on postnatal day 21 (P21). WT mice used for controls were subjected to the same corticosteroid treatment protocol as SF-1 KO mice. After weaning, mice of the same chromosomal sex were group housed (2 to 3 per cage) until the time of sacrifice (gonadectomized WT and SF-1 KO mice were never housed with the gonadally intact WT mice, due to the possible aggressive behavior by WT males).
Some WT mice were gonadectomized before puberty (P21) to prepare controls with a comparable gonadal status to SF-1 KO mice. For gonadectomies, WT mice were anesthetized with a mixture of ketamine (Vetoquinol Biowet, Gorzowie, Poland; 100 μg/g BW), xylazine (Chanelle Pharmaceuticals Ltd., Loughrea, Ireland; 10 μg/g BW) and acepromazine (Fort Dodge Animal Health, Fort Dodge, IA, USA; 2 μg/g BW) and ovaries and testes were removed through a single or bilateral incision, respectively. After gonadectomy, mice received two injections of the analgesic butorphanol (Fort Dodge Animal Health; 1.7 μg/g BW). To control for gonadectomy, gonadally intact WT and SF-1 KO mice were sham operated at P21.
To study the effect of EB exposure during a pubertal time period, SF-1 KO and gonadectomized WT mice were anesthetized with a mixture of ketamine, xylazine and acepromazine and implanted subcutaneously with silastic capsules (1.02 mm i.d. × 2.16 mm o.d.; Dow Corning, MI, USA; 5 mm of tubing filled with EB and cholesterol (Sigma) in a ratio 1:1) for a period from P25 to P36 as reported previously [58].
At the time of sacrifice, mice were anesthetized as described above and perfused with 4% paraformaldehyde (Sigma) in 0.1M PB, pH = 7.4. All adult gonadally intact WT females were sacrificed in diestrus, based on vaginal smear cytology. After removal, brains remained in the same fixative overnight at 4°C and then stored until immunocytochemical processing in 0.1M PB at 4°C.
Puberty was determined by monitoring vaginal opening in a separate cohort of 10 WT and SF-1+/- female mice. Timing of vaginal opening ranged from P34 to P38 with most female mice in the colony presenting with vaginal opening by P36.
Experimental design
To study the effects of sex chromosomes and gonadal steroids on kisspeptin sexual differentiation in the brain, SF-1 KO mice (M: n= 18, F: n= 19) were compared to gonadally intact (M: n= 19, F: n= 18) or gonadectomized (M: n= 9, F: n= 10) control WT mice at three different developing stages: before puberty at P25, approximately during puberty at P36 and in young adulthood at P60. The effect of EB on kisspeptin brain sexual differentiation was studied in gonadectomized WT mice (M: n= 5, F: n= 6) and SF-1 KO mice (M: n= 6, F: n= 5) that were implanted with EB silastic capsules from P25 to P36 (during a pubertal period) and sacrificed at P36.
Immunohistochemistry on floating brain sections
Brains were embedded in 5% agarose (Sigma) and sectioned at 50 μm in cold 0.05 M PBS using a vibrating microtome (Integraslice 7550 MM, Campden Instruments, UK). Sections were placed in alternating containers to aid free floating tissue processing and to help track locations more readily. Before primary antibodies, sections were incubated in 0.1 M glycine (Sigma) and 0.5% sodium borohydride (Sigma) in 0.05 M PBS for 30 min and 15 min at 4°C, respectively. Sections were then blocked in 5% normal goat serum (Chemicon, Temecula, CA, USA) containing 0.5% Triton X-100 (Sigma) and 1% H2O2 (Merck, Darmstadt, Germany) for 30 min at 4°C. Sections were incubated in rabbit primary antiserum against kisspeptin 10 (gift from Caraty A., this antiserum has been used and validated before, [16]) diluted 1: 30,000 in 0.05 M PBS containing 1% bovine serum albumin (Sigma) and 0.5% Triton X-100 over 3 nights at 4°C with shaking. Sections were then washed in 0.05 M PBS containing 1% normal goat serum and 0.02% Triton X-100 four times 15 minutes at room temperature. Biotinylated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) against primary rabbit antisera were diluted 1:500 in 0.05 M PBS containing 1% normal goat serum and 0.5% Triton X-100. Sections were incubated with secondary antibodies for two hours, followed by 4 washes (15 minutes each) in 0.05 M PBS buffer containing 0.02% Triton X- 100. Streptavidin – HRP complex (Jackson Immunoresearch) was diluted 1:2500 in 0.05 M PBS solution containing 0.5% TritonX-100. Sections were incubated with Streptavidin conjugated horseradish peroxidase for 1 hour at room temperature and then washed in Tris-buffered saline (0.05 M Tris-HCl/0.9% NaCl; pH 7.5; Sigma) for 1 hour at room temperature. Antigen-antibody complexes were visualized as a black reaction product by incubating sections in 0.025% 3′3′-Diaminobenzidine/ammonium nickel (II) sulfate substrate (Sigma) in Tris-buffered saline (pH 7.5) containing 0.02% H2O2 for 5 min at room temperature. After mounting on slides, sections were dried and coverslipped using hydrophobic medium (Pertex, Burgdorf, Germany). Immunocytochemical controls were based on omitting primary antibodies from control sections and on a validation of immunoreactivity with patterns of distribution from a prior report [16].
Data collection and analyses
Digital images of the AVPV region were obtained using a Nikon Eclipse 80i microscope with Nikon DS-Fi1 camera. Kisspeptin-ir cells in the AVPV region were counted directly under the microscope in coronal sections considered in order from rostral to caudal on the microscopic slides. Cells were counted on both left and right side of the AVPV on two sections that always corresponded to sections approximately 0.14 mm and 0.26 mm rostral from bregma according to stereotaxic coordinates [59]. Kisspeptin immunoreactivity on both left and right side of the Arc was quantified in coronal sections (100× magnification; the third ventricle and base of the brain as reference boundaries) corresponding to sections approximately 1.7 mm caudal from bregma according to stereotaxic coordinates [59] using a custom software (Surfkvad; made by Dr. Marko Kreft, Institute of Pathophysiology, Faculty of Medicine, Ljubljana). For the purpose of analysis with Surfkvad, all digital images were taken under the same illumination settings and were prepared accordingly using Adobe Photoshop software package (Version 8.0) as follows; digital images were standardized for illumination, which eliminated the possible differences in the intensity of the background between images, converted to grayscale and then subjected to threshold conversion to selectively identify immunoreactive elements with threshold limit set to 50%. Black and white images were then analyzed with Surfkvad software that calculates the percentage of dark area (immunoreactivity) in a boxed area of interest (940 × 970 μm) bounded by the third ventricle medially and the base of the brain.
Statistical analysis
Statistical analysis was performed using the NCSS software package (NCSS 2007, Kaysville, UT). Statistical differences in the number of kisspeptin-ir cells in the AVPV and the kisspeptin-ir area in the Arc between gonadally intact WT mice, gonadectomized WT mice and SF-1 KO mice were examined by three-factor ANOVA with sex, genotype/gonadal status and age as independent factors. Differences in the number of kisspeptin-ir cells in the AVPV between different developing stages in gonadally intact WT male and female mice were examined in a planned comparison by two- factor ANOVA with sex and age as independent factors. Differences in the kisspeptin-ir area between different developmental stages in gonadally intact WT and SF-1 KO mice were examined in a planned comparison with sex, genotype and age as independent factors. Differences in the kisspeptin-ir area between gonadally intact WT, gonadectomized WT and SF-1 KO mice at P36 only were examined in a planned comparison by three-factor ANOVA with sex, genotype and gonadal status as independent factors. The effect of EB on the number of kisspeptin-ir cells in the AVPV and the kisspeptin-ir area in the Arc was examined in the 36 day old mice only by three-factor ANOVA with genotype/gonadal status, sex and treatment as independent factors. When applicable, Fisher's LSD post hoc analyses were used to determine statistical differences between groups. Statistical differences were considered significant with p < 0.05. All data are presented as mean +/- SEM.
Results
Lack of SF-1 did not result in WT female levels of kisspeptin immunoreactive cells in the AVPV in the absence of gonadal steroids
As expected, kisspeptin-ir cells in the AVPV were detected in the periventricular area (Fig. 1). The number of kisspeptin-ir cells in the AVPV increased with age from P25 to P60 and reached a maximum at P60 (F (4, 103)= 25.73; p< 0.001, Fisher's LSD; p< 0.05) in gonadally intact WT male and female mice in a sex specific manner (F (2, 103)= 81.61; p< 0.001, Fisher's LSD; p< 0.05). Furthermore, a planned comparison showed that the number of kisspeptin-ir cells significantly (F (2, 35)= 17.68; p< 0.001, Fisher's LSD; p< 0.05) increased from P25 to P36 and from P36 to P60 in gonadally intact WT females but not males, which led to higher (F (2, 35)= 17.68; p< 0.001, Fisher's LSD; p< 0.05) kisspeptin-ir cell numbers in WT females than WT males at P25, P36 and P60. (Fig.1). Gonadectomy of WT mice before puberty at P21 led to significantly reduced numbers of kisspeptin-ir cells in males (WT/CAS) and females (WT/OVX) in comparison to gonadally intact WT mice (F (2, 103)= 81.61; p< 0.001, Fisher's LSD p< 0.05; Fig.1). In gonadectomized WT mice, a sex difference in the number of kisspeptin-ir cells was conserved at P25 (p< 0.05, Fisher's LSD), but not at P36 and P60 when kisspeptin-ir cells were not detected in this area in either sex (Fig. 1). In agonadal SF-1 KO mice, the total number of kisspeptin-ir cells was indistinguishable (p> 0.05, Fisher's LSD) from that of gonadally intact/gonadectomized WT males regardless of sex at all three ages studied (Fig. 1), and did not differ (p> 0.05, Fisher's LSD) from WT/OVX females at P60.
Fig. 1.
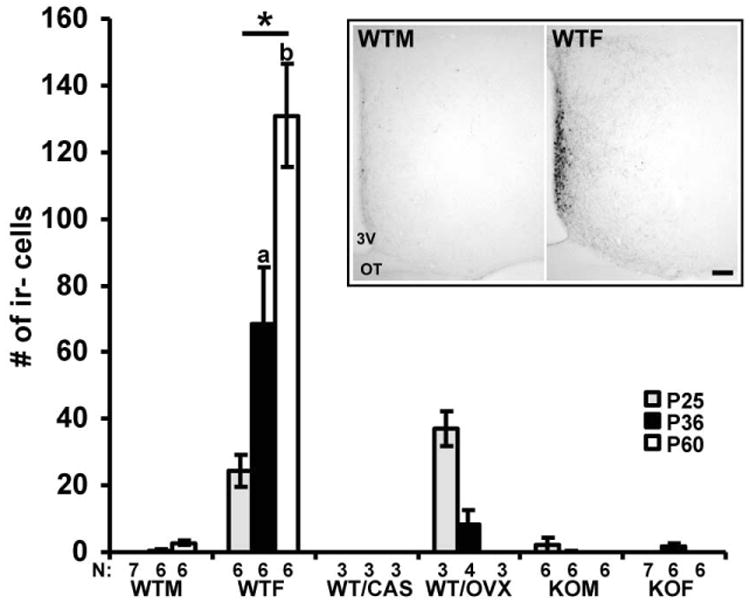
A digital inset image shows representative sections with kisspeptin-ir cells in the AVPV at P60 in WTM and WTF mice. The graph illustrates the number of kisspeptin-ir cells in the AVPV in gonadally intact wild type (WT) male (WTM) and female (WTF), gonadectomized at P21 WT male (WT/CAS), and female (WT/OVX), and agonadal SF-1 KO male (KOM) and female (KOF) mice in three different developmental stages, at P25 (before puberty), at P36 (during pubertal time) and at P60 (adult). The number of cells is presented as mean +/- SEM. *p < 0.05 (significantly different from the WTM, WT/CAS, WT/OVX, KOM and KOF); ap< 0.05 (significantly different from WTF at P25); bp< 0.05 (significantly different from WTF at P36). For the image, 3V- third ventricle, OT- optic tract, bar: 100 μm.
Lack of SF-1 did not result in female levels of kisspeptin immunoreactive cells in the AVPV in the presence of gonadal steroids
To examine the effect of exposure to EB on the number of kisspeptin-ir cells in the AVPV during puberty, gonadectomized WT and agonadal SF-1 KO mice were exposed to EB from P25 to P36. EB treatment increased (F(1,55)= 23.29; p< 0.001) the total number of kisspeptin-ir cells in both WT/GDX and SF-1 KO mice. Significant interaction (sex × genotype/gonadal status × treatment; F(2,55)= 4.37; p< 0.05) suggests EB treatment from P25 to P36 restored the total number of kisspeptin-ir cells to WT female levels only in WT/OVX mice, but surprisingly, not in agonadal EB treated SF-1 KO male and female mice that had significantly (F(2,55)= 20.86; p< 0.001, Fisher's LSD; p< 0.05) fewer kisspeptin-ir cells than EB treated WT/OVX and untreated gonadally intact WT female mice (Fig. 2).
Fig. 2.
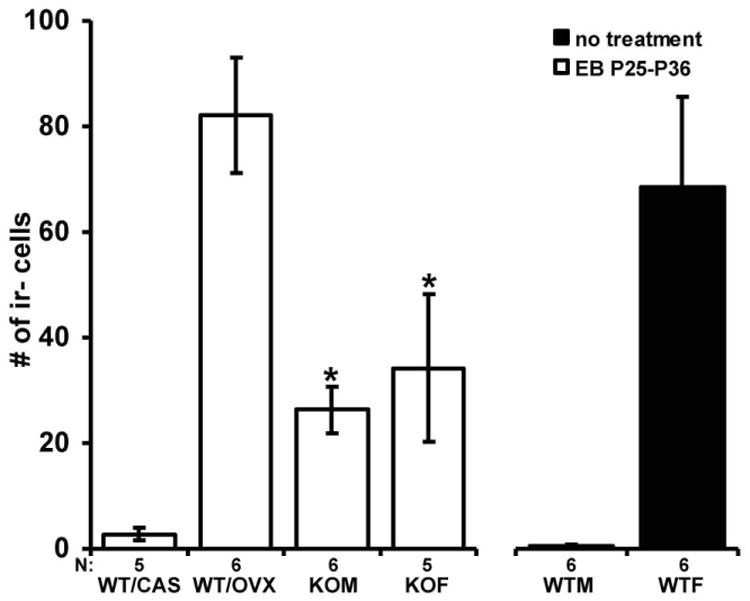
The diagram illustrates the number of kisspeptin-ir cells in the AVPV from 36 days old gonadally intact wild type male (WTM) and female (WTF) mice (same bars as in diagram 1, included for comparison), WT male (WT/CAS) and female (WT/OVX) mice gonadectomized at P21 and SF-1 KO male (KOM) and female (KOF) mice. Gonadectomized mice and agonadal SF-1 KO mice were treated with EB from P25 to P36. Treatment restored the number of kisspeptin-ir cells in WT/OVX mice to the levels seen in WTF mice, while the number of kisspeptin-ir cells in agonadal SF-1 KO mice of both sexes was significantly lower (p< 0.05) in comparison to WTF mice even after EB treatment. The number of cells is presented as mean +/- SEM. *p < 0.05, significantly lower than EB treated WT/OVX mice and untreated WTF mice.
Lack of SF-1 did not prevent sexually dimorphic kisspeptin immunoreactivity in the Arc at the time of puberty onset in the absence of gonadal steroids
Kisspeptin-ir area in the Arc increased with age from P25 to P60 (reaching a maximum at P60 (F (4, 92)= 7.97; p< 0.001, Fisher's LSD; p< 0.05)) in gonadally intact WT mice in a sex specific manner (F (2, 92)= 23.51; p< 0.001, Fisher's LSD; p< 0.05) with gonadally intact WT females having significantly higher immunoreactive area than males at all three ages studied (F (4, 92)= 3.09; p< 0.05, Fisher's LSD; p< 0.05). Overall, gonadectomy at P21 significantly decreased (F (2, 92)= 26.87; p< 0.001) kisspeptin-ir area in comparison to gonadally intact WT mice in a sex dependent manner with WT/OVX having significantly higher (F (2, 92)= 23.51; p< 0.001, Fisher's LSD; p< 0.05) kisspeptin-ir area than WT/CAS mice (Fig. 3).
Fig. 3.
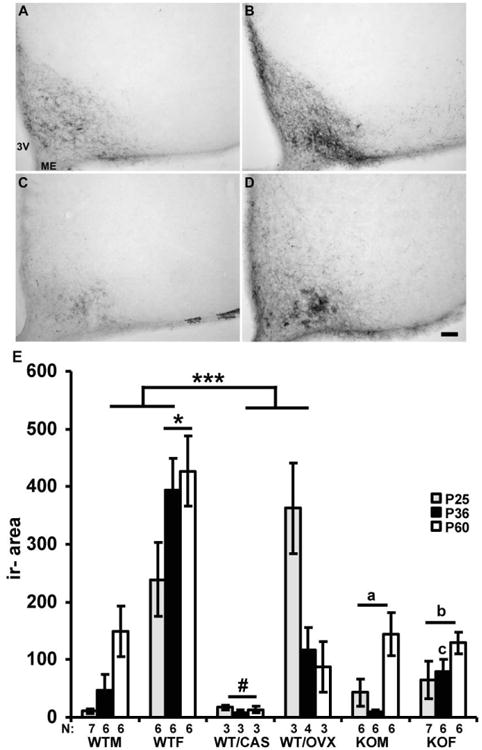
A- D] Representative images of kisspeptin immunoreactivity in the Arc at P36 in gonadally intact WT male (A) and female (B) and agonadal SF-1 KO male (C) and female (D) mice. Gonadally intact WT and agonadal SF-1 KO female mice had significantly higher kisspeptin-ir area in the Arc during puberty at P36 than gonadally intact WT and agonadal SF-1 KO males respectively. E] The graph illustrates the kisspeptin-ir area in the Arc in gonadally intact wild type (WT) male (WTM) and female (WTF), gonadectomized at P21 WT male (WT/CAS), and female (WT/OVX), and agonadal SF-1 KO male (KOM) and female (KOF) mice in three different developmental stages, at P25 (before puberty), at P36 (during pubertal time) and at P60 (adult). The kisspeptin-ir area is presented as mean +/- SEM. *p< 0.05 (different from the WTM, WT/CAS, WT/OVX and SF-1 KO mice over all ages studied); ***p< 0.001 (higher immunoreactive area in WT than gonadectomized WT mice); #p< 0.05 (different from WT OVX); ap< 0.05 (different from WTF and WT/OVX mice); bp< 0.05 (different from WTF, WT/OVX and WT/CAS mice) cp< 0.05 (higher than KOM at P36). 3V- third ventricle, ME- median eminence, bar: 100 μm
Kisspeptin-ir area in the Arc was lower in SF-1 KO male and female mice than in gonadally intact WTF and WT/OVX mice (F (2, 92)= 23.51; p< 0.001, Fisher's LSD; p< 0.05) at all ages studied (Fig. 3) and this was due to the substantial increase in WT/OVX observed at P25, which may reflect the increased kisspeptin immunoreactivity after gonadectomy as reported before [12]. Later at P36 and P60 the kisspeptin-ir area in WT/OVX mice was within the range of immunoreactivity found in agonadal SF-1 KO mice. However, agonadal SF-1 KO female mice had significantly higher immunoreactive area in the Arc than WT/CAS mice (F (2, 92)= 23.51; p< 0.001, Fisher's LSD; p< 0.05) (Fig. 3).
Interestingly, kisspeptin -ir area in gonadally intact WT females and SF-1 KO females reached adult levels already at P36, while in gonadally intact WT males and SF-1 KO males, the ir area at P36 was lower (p< 0.05; Fisher's LSD) than that at P60 (Fig. 3). This suggests later maturation of immunoreactive kisspeptin levels in gonadally intact WT and SF-1 KO males in comparison to females, and this is likely regulated by gonad independent factors as it is conserved in SF-1 KO mice.
Sexually dimorphic kisspeptin immunoreactivity in the Arc was eliminated in the presence of sex steroid hormones in mice lacking SF-1
To examine the effect of sex steroid hormones on immunoreactive kisspeptin levels after gonadectomy, WT mice gonadectomized at P21 and SF-1 KO mice were treated with EB from P25 to P36 and examined at P36. Analysis did not reveal any statistically significant effect of EB treatment on kisspeptin-ir area although interestingly, slight increases in immunoexpression in SF-1 KO males and slight decreases in kisspeptin immunoexpression in SF-1 KO females, respectively eliminated the sex difference observed in hormonally naïve SF-1 KO mice at P36 (Fig. 4). In gonadectomized WT mice kisspeptin-ir area remained higher (F (1,52)= 44.08; p< 0.001, Fisher's LSD; p< 0.05) in females than males.
Fig. 4.
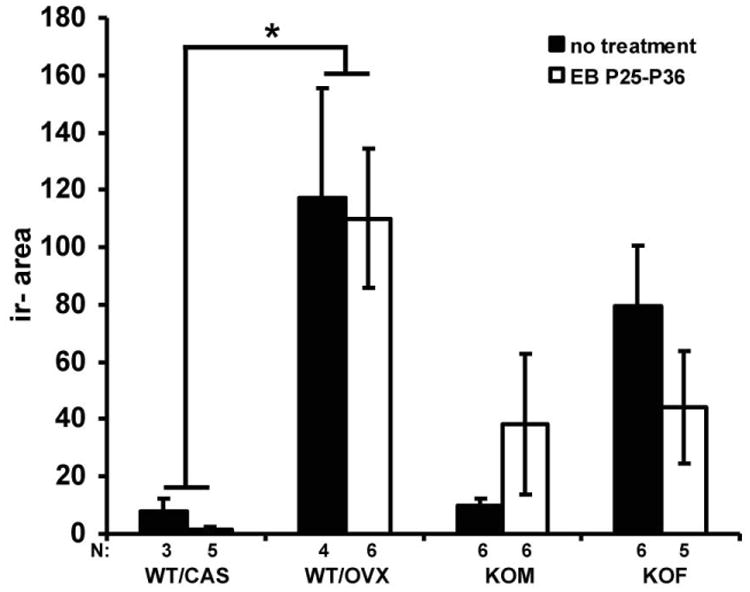
The diagram illustrates the kisspeptin-ir area in the Arc from 36 days old wild type male (WT/CAS) and female (WT/OVX) mice gonadectomized at P21 and SF-1 KO male (KOM) and female (KOF) mice. All mice were untreated or treated with EB from P25 to P36. EB treatment from P25 to P36 had no effect on the kisspeptin-ir area in both WT/CAS and WT/OVX mice when compared to untreated gonadectomized WT mice. However, EB treatment did eliminate the sex difference in agonadal SF-1 KO mice at P36 by moderately (nonsignificantly) increasing immunoexpression in males and decreasing immunoexpression in females. The kisspeptin-ir area is presented as mean +/- SEM. *p< 0.05 (different from WT/CAS).
Discussion
Neurons expressing the Kiss1 gene and its receptor have a major role in the regulation of GnRH release in the hypothalamus and in the onset of puberty, as evidenced by studies in mice [2,9,60] and humans [1,3]. In the murine brain, expression of Kiss1 is sex-dependent, and this sex difference is usually considered a result of exposure to sex steroid hormones, especially neonatally in male rats [15], but also prenatally in mice [27]. Previous studies suggest that the number of Kiss1 mRNA expressing neurons [61] and kisspeptin-ir neurons [20] reaches adult levels by the time of puberty and that EB treatment before/during puberty is sufficient for the full restoration of these levels in the AVPV in ovariectomized WT mice [20]. In the present study, the kisspeptin-ir neural population in the AVPV was therefore examined in agonadal SF-1 KO mice during postnatal development and after treatment with EB during the pubertal period. If perinatal gonadal steroids are sufficient to cause a masculine pattern of kisspeptin expression in adult mice, then in agonadal SF-1 KO mice (regardless of genetic sex) kisspeptin-ir should follow a female pattern of expression after treatment with EB. The results of the current study, however, show a diminished number of cells containing immunoreactive kisspeptin in untreated agonadal SF-1 KO mice from P25 to early adulthood. Treatment of WT/OVX mice with EB from P25 to P36 restored the number of kisspeptin-ir cells to the levels found in gonadally intact WT female mice at P36 in the AVPV. In contrast, similar treatment of SF-1 KO mice of both sexes did not produce similar numbers of kisspeptin-ir cells. An analysis of the significant interaction between genotype/gonadal status, sex and treatment suggests that treatment from P25 until P36 was not sufficient for full induction of immunoreactive kisspeptin in cells in the AVPV in SF-1 KO mice and that either earlier programming of this response is needed, or some other factor(s) besides estradiol are needed for full induction of immunoreactive kisspeptin in the AVPV. Such programming could be caused by earlier exposure to gonadal steroid hormones, or some other gonadal factors, that are absent in SF-1 KO mice due to early gonadal agenesis. These results are similar in part to results from mice lacking the aromatase enzyme (ARKO mice) [23]. In that study, postnatal or adult treatments with EB induced expression of kisspeptin in the hypothalamus of male and female ARKO mice (that normally express very low levels of kisspeptin), but the number of cells in ARKO mice was lower than in WT mice even after treatment with EB, suggesting an earlier organizational period, probably dependent on ovarian steroid hormones [23]. In agreement, another report [21] indicated that exposure to estradiol during an earlier juvenile period, and not just pubertal as suggested in some previous studies [20], is likely needed for full feminization of the kisspeptin system in the AVPV region. Taken together, it seems likely that there are additional factors, perhaps an early exposure to low levels of ovary-derived estrogens between P10 and P15, that are indispensable for the development of Kiss1 neurons in the AVPV in female mice, and possibly some other female specific factors [23,62-64].
In the present study, immunoreactive kisspeptin in Arc fibers was followed from before puberty (P25) to after puberty (P60) in gonadally intact/gonadectomized WT and agonadal SF-1 KO mice. Previous studies have shown that besides gonadal steroid hormones [12-15,19,23], Kiss1 expression at the mRNA and peptide level in the Arc is also regulated by various biological molecules including neuropeptides (dynorphin A and neurokinin B [34,35], prolactin [37]), neurotransmitters (GABA and glutamate [43,44]), trophic factors (LRH1 [42], insulin [38], mTOR signaling [39]) and metabolic factors (leptin [36], ghrelin [41] and NPY [40]). Further, functional changes in Kiss1 mRNA expression in the Arc associated with puberty control independent of gonadal hormones were reported to depend on PcG group of transcriptional silencers [33]. The kisspeptin-ir area in adult gonadally intact WT mice was established through a gradual increase starting from P25 in both WT male and female mice, with females having greater immunoreactive area than males at all ages studied. Kisspeptin-ir area in the Arc in agonadal SF-1 KO mice did not differ between sexes at P25 and P60, but there was a significant difference between SF-1 KO males and females during puberty at P36. While gonadectomy decreased kisspeptin immunoreactivity in the Arc in both males and females in comparison to gonadally intact WT mice, it did not eliminate sex differences in the area of kisspeptin-ir. Similarly as in intact WT mice, gonadectomized WT females had significantly greater area containing kisspeptin-ir than gonadectomized WT males at all ages studied. These results suggest that sexual differentiation of the kisspeptin system in the Arc during postnatal development in mice depends on gonadal hormone dependent factors. However, our results also suggest that sexual differentiation of the kisspeptin system during puberty differs between males and females, and this depends on additional regulatory mechanisms, provided by gonadal- independent factors, as is observed in agonadal SF-1 KO mice.
Functional reactivation of GnRH neurons and consequent activation of the HPG axis represents a hallmark for the beginning of puberty that occurs earlier in girls than boys. Although dealing with the regulation of gonadal hormone synthesis and almost complete dependence of Kiss1 mRNA and kisspeptin expression on gonadal hormones, it is interesting that some changes in gene expression controlling GnRH neuron activity occur independently of circulating gonadal levels. For example, increases in Kiss1 mRNA expression in the Arc during puberty occur in male mice gonadectomized at P14 [65] and in hpg male and female mice [32]. Observations that the HPG axis becomes responsive to Kp-54 at P30 in rats and not earlier [66] further suggest regulation of GnRH neuron reactivation by intrinsic biological clocks. The current study extends these observations by showing sex- specific increases in the area of kisspeptin-ir in the Arc during puberty in agonadal SF-1 KO mice. At the time of puberty, agonadal SF-1 KO female mice had significantly greater area containing kisspeptin-ir than agonadal SF-1 KO male mice. The levels of immunoreactive kisspeptin were maximal in agonadal SF-1 KO female mice already at P36 while there was a significant increase between days 36 and 60 in SF-1 KO males, suggesting that males reach adult pattern of expression later than females, possibly due to later pubertal development. Despite the differences in the total area of kisspeptin-ir between agonadal SF-1 KO and gonadally intact WT mice, similar developmental dynamics in the area of kisspeptin-ir to that in agonadal SF-1 KO mice was observed in gonadally intact WT mice. The results of the current study suggest that sexually dimorphic timing in changes of the area of kisspeptin-ir during puberty is regulated by gonad- independent factors.
Sex differences in kisspeptin expression in the Arc during early postnatal development in mice to some extent depends on gonadal hormones [23]. In the current study, gonadectomy before puberty at P21 decreased but did not eliminate the kisspeptin-ir in WT male and female mice later during puberty (P36) and in early adulthood (P60) in a sex specific manner. However, treatment with EB during puberty from P25 to P36 did not restore the kisspeptin-ir area in gonadectomized WT mice to the levels in gonadally intact WT mice suggesting that other gonadal hormones (perhaps testosterone in males [13], progesterone in females) and/ or other yet unknown factors regulate the levels of immunoreactive kisspeptin during puberty in the Arc. This is in contrast to a study in ARKO mice that reported complete restoration of kisspeptin immunoreactivity in the Arc in adult WT and ARKO mice after 10 days of treatment with estradiol or dihydrotestosterone [23]. However, mice in that study were left gonadally intact during puberty and gonadectomized only in adulthood, which might impact the Arc Kiss1 circuitry differently than prepubertal gonadectomy. Furthermore, mice in the present study were not treated with colchicine before sacrifice therefore the effect of EB on an increased area of kisspeptin-ir could be masked by the increased kisspeptin transport and secretion. Kisspeptin expression in the Arc was studied by immunohistochemistry, which did not allow us to determine whether there were other factors regulating the amount of immunoreactive kisspeptin in fibers. Therefore, the current data (area of kisspeptin-ir) only provides information about the quantity of immunoreactive kisspeptin in fibers in the Arc at the time of sacrifice.
In conclusion, the results of the current study show that EB treatment from P25 to P36 could not restore the kisspeptin-ir cell numbers in AVPV in agonadal SF-1 KO mice to numbers found in gonadally intact WT or EB treated WT/OVX females. This suggests that either earlier programming by sex steroids (before P21) is needed for normal expression of kisspeptin in the AVPV, or that other factor(s) besides estradiol, either at puberty or during earlier development, are needed for the capacity to express kisspeptin in the AVPV. Furthermore, results from the Arc analyses suggest that maturation of immunoreactive kisspeptin in the Arc occurs at different ages in males and females, and this sex difference is independent of gonads as it was observed also in agonadal SF-1 KO mice.
Acknowledgments
We would like to thank Dr. Alain Caraty for contributing kisspeptin antibodies and Ms. Nina Sterman for animal husbandry and technical assistance. This study was supported by NIH R01 MH61376 (SAT, GM) and ARRS P4-0053 (GM, TB).
References
- 1.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 3.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 4.Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol. 2006;18:349–354. doi: 10.1111/j.1365-2826.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- 5.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 6.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 7.Tovar S, Vazquez MJ, Navarro VM, Fernandez-Fernandez R, Castellano JM, Vigo E, Roa J, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology. 2006;147:2696–2704. doi: 10.1210/en.2005-1397. [DOI] [PubMed] [Google Scholar]
- 8.Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- 9.d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–3370. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- 11.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 13.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 14.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 15.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 16.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarkson J, Shamas S, Mallinson S, Herbison AE. Gonadal steroid induction of kisspeptin peptide expression in the rostral periventricular area of the third ventricle during postnatal development in the male mouse. J Neuroendocrinol. 2012;24:907–915. doi: 10.1111/j.1365-2826.2012.02294.x. [DOI] [PubMed] [Google Scholar]
- 19.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81:1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150:3214–3220. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Tolson KP, Dhamija S, Kauffman AS. Developmental GnRH signalling is not required for sexual differentiation of kisspeptin neurons but is needed for maximal Kiss1 gene expression in adult females. Endocrinology. 2013;154:3273–3283. doi: 10.1210/en.2013-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology. 2013;154:2739–2749. doi: 10.1210/en.2013-1120. [DOI] [PubMed] [Google Scholar]
- 24.Fiorini Z, Jasoni CL. A novel developmental role for kisspeptin in the growth of gonadotrophin-releasing hormone neurites to the median eminence in the mouse. J Neuroendocrinol. 2010;22:1113–1125. doi: 10.1111/j.1365-2826.2010.02059.x. [DOI] [PubMed] [Google Scholar]
- 25.Desroziers E, Droguerre M, Bentsen AH, Robert V, Mikkelsen JD, Caraty A, Tillet Y, Duittoz A, Franceschini I. Embryonic development of kisspeptin neurones in rat. J Neuroendocrinol. 2012;24:1284–1295. doi: 10.1111/j.1365-2826.2012.02333.x. [DOI] [PubMed] [Google Scholar]
- 26.Desroziers E, Mikkelsen JD, Duittoz A, Franceschini I. Kisspeptin-immunoreactivity changes in a sex- and hypothalamic-region-specific manner across rat postnatal development. J Neuroendocrinol. 2012;24:1154–1165. doi: 10.1111/j.1365-2826.2012.02317.x. [DOI] [PubMed] [Google Scholar]
- 27.Knoll JG, Clay CM, Bouma GJ, Henion TR, Schwarting GA, Millar RP, Tobet SA. Developmental profile and sexually dimorphic expression of kiss1 and kiss1r in the fetal mouse brain. Front Endocrinol (Lausanne) 2013;4:140. doi: 10.3389/fendo.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci. 2011;43:138–145. doi: 10.1007/s12031-010-9430-1. [DOI] [PubMed] [Google Scholar]
- 29.Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009;30:350–357. doi: 10.1016/j.neuro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conte FA, Grumbach MM, Kaplan SL. A diphasic pattern of gonadotropin secretion in patients with the syndrome of gonadal dysgenesis. J Clin Endocrinol Metab. 1975;40:670–674. doi: 10.1210/jcem-40-4-670. [DOI] [PubMed] [Google Scholar]
- 31.Andrews WW, Advis JP, Ojeda SR. The maturation of estradiol-negative feedback in female rats: evidence that the resetting of the hypothalamic “gonadostat” does not precede the first preovulatory surge of gonadotropins. Endocrinology. 1981;109:2022–2031. doi: 10.1210/endo-109-6-2022. [DOI] [PubMed] [Google Scholar]
- 32.Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One. 2010;5:e11911. doi: 10.1371/journal.pone.0011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, Knoll JG, Wright H, Pfeifer GP, Ojeda SR. Epigenetic control of female puberty. Nat Neurosci. 2013;16:281–289. doi: 10.1038/nn.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 35.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 37.Araujo-Lopes R, Crampton JR, Aquino NS, Miranda RM, Kokay IC, Reis AM, Franci CR, Grattan DR, Szawka RE. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014;155:1010–1020. doi: 10.1210/en.2013-1889. [DOI] [PubMed] [Google Scholar]
- 38.Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Bruning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roa J, Garcia-Galiano D, Varela L, Sanchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, Lopez M, Gaytan F, Dieguez C, Pinilla L, Tena- Sempere M. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150:5016–5026. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- 40.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- 41.Forbes S, Li XF, Kinsey-Jones J, O'Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett. 2009;460:143–147. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 42.Atkin SD, Owen BM, Bookout AL, Cravo RM, Lee C, Elias CF, Elmquist JK, Kliewer SA, Mangelsdorf DJ. Nuclear receptor LRH-1 induces the reproductive neuropeptide kisspeptin in the hypothalamus. Mol Endocrinol. 2013;27:598–605. doi: 10.1210/me.2012-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 44.d'Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, Colledge WH. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci. 2010;30:8581–8590. doi: 10.1523/JNEUROSCI.5486-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrenker P, Maxson SC. The genetics of hormonal influences on male sexual behavior of mice and rats. Neurosci Biobehav Rev. 1983;7:349–359. doi: 10.1016/0149-7634(83)90037-4. [DOI] [PubMed] [Google Scholar]
- 46.De Vries GJ. Sex steroids and sex chromosomes at odds? Endocrinology. 2005;146:3277–3279. doi: 10.1210/en.2005-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Front Neuroendocrinol. 2011;32:137–145. doi: 10.1016/j.yfrne.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 50.Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154:1501–1512. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grgurevic N, Budefeld T, Spanic T, Tobet SA, Majdic G. Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior. Horm Behav. 2012;61:719–724. doi: 10.1016/j.yhbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 55.Budefeld T, Jezek D, Rozman D, Majdic G. Initiation of steroidogenesis precedes expression of cholesterologenic enzymes in the fetal mouse testes. Anat Histol Embryol. 2009;38:461–466. doi: 10.1111/j.1439-0264.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- 56.Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 57.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 58.Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- 59.Paxinos G, Franklin KBJ. The Mouse Brain in the Stereotaxic Coordinates. 2. San Diego, San Francisco, New York, Boston, London, Sydney, Tokyo: Academic Press; 2001. [Google Scholar]
- 60.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84–97. doi: 10.1016/j.mce.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerall AA, Dunlap JL. Evidence that the ovaries of the neonatal rat secrete active substances. J Endocrinol. 1971;50:529–530. doi: 10.1677/joe.0.0500529. [DOI] [PubMed] [Google Scholar]
- 63.Dunlap JL, Gerall AA, Carlton SF. Evaluation of prenatal androgen and ovarian secretions on receptivity in female and male rats. J Comp Physiol Psychol. 1978;92:280–288. doi: 10.1037/h0077462. [DOI] [PubMed] [Google Scholar]
- 64.Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25:2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297:E1212–1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bentsen AH, Ansel L, Simonneaux V, Tena-Sempere M, Juul A, Mikkelsen JD. Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides. 2010;31:275–283. doi: 10.1016/j.peptides.2009.11.017. [DOI] [PubMed] [Google Scholar]


