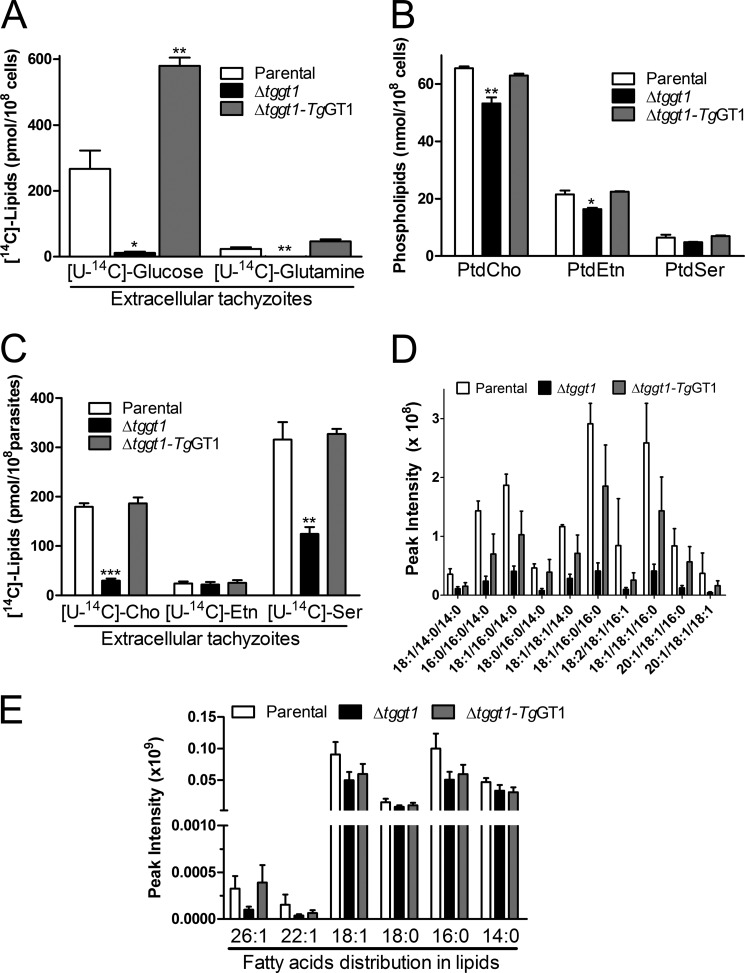
FIGURE 6.
A dysfunctional glycolysis is detrimental to the membrane biogenesis. A, metabolic labeling of nascent lipids using [U-14C]glucose or [U-14C]glutamine in extracellular parasites. Total parasite lipids were prepared to determine the radiolabeling by liquid scintillation counting. B, comparative amounts of three major lipids in the depicted strains. Phospholipids extracted from extracellular parasites were resolved by thin layer chromatography, detected by iodine-vapor staining, and quantified by chemical-phosphorous assay (see supplemental Fig. S8 for TLC images). PtdCho, phosphatidylcholine, PtdEtn, phosphatidylethanolamine, PtdSer, phosphatidylserine. C, radiolabeling of parasite lipids with choline, ethanolamine, or serine. Extracellular tachyzoites were incubated with either of the U-14C-labeled head groups and radiotracer incorporated into total lipids was measured. The data plotted in panels A–C show mean ± S.E. from three assays. D, relative contents of the major triacylglycerol species in the three parasite strains, as monitored by lipidomics analysis. Lipids isolated from fresh extracellular parasites were analyzed by ultra-performance liquid chromatography-mass spectrometry (mean ± S.E., n = 5). E, estimated amounts of acyl chains conjugated to total lipids in specified strains. Intensities on the y axis denote cumulative sum of all peaks corresponding to a given acyl chain irrespective of the bound lipid (mean ± S.E., n = 5). Lipids bound to the specific acyl chains were quantified from the areas of chromatographic peaks. Statistical significance was measured separately for each group with respect to the parental strain using Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Note that the Δtggt1 mutant in panels D–E did not show any significant difference for individual lipid species; however, a collective reduction across all lipid species is very significant (two-way analysis of variance) (panel D, p < 0.0001; panel E, p = 0.0060).