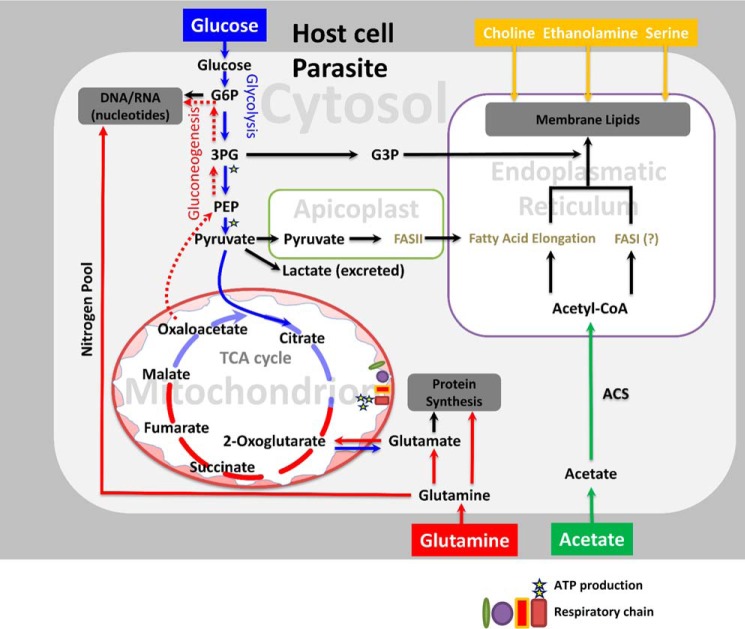
FIGURE 8.
Carbon metabolism of T. gondii converges with tumor cells. Proposed model of central carbon metabolism is constructed based on this work, published literature, and annotations of select enzymes expressed in the tachyzoite stage (ToxoDB). Only those metabolites detectable or relevant to this work are shown for simplicity. Glucose and glutamine are co-utilized to satisfy the demands of biomass (proteins, nucleotides, lipids), energy, and reducing equivalents (not depicted). Nucleotides synthesis requires ribose 5-phosphate produced by diversion of glycolytic metabolites to the pentose phosphate shunt. Lipid biogenesis utilizes acetyl-CoA and glycerol-3-phosphate, which are primarily derived from glucose under normal condition. Likewise, protein synthesis needs glucose-derived amino acids. When replicating intracellular, glutamine catabolism enables an efficient biosynthetic use of glucose by replenishing the TCA cycle metabolites drained by biogenesis of macromolecules. Glutamine also confers the much-needed pool of nitrogen for nucleotide and protein syntheses. Extracellular parasites can use either of the two nutrients to produce sufficient energy for the host-cell invasion. Glutamine-derived carbon flux (TCA cycle and gluconeogenesis) sustains the parasite survival with a minimal growth defect when glycolysis is compromised. The parasite can also deploy acetate as a carbon source when available in culture. Carbon metabolism is reprogrammed according to proliferating (intracellular) and non-proliferating (extracellular) demands and in response to the available nutrients. G6P, glucose-6-phosphate; 3PG, 3-phosphoglycerate; G3P, glycerol-3-phosphate; PEP, phosphoenolpyruvate.