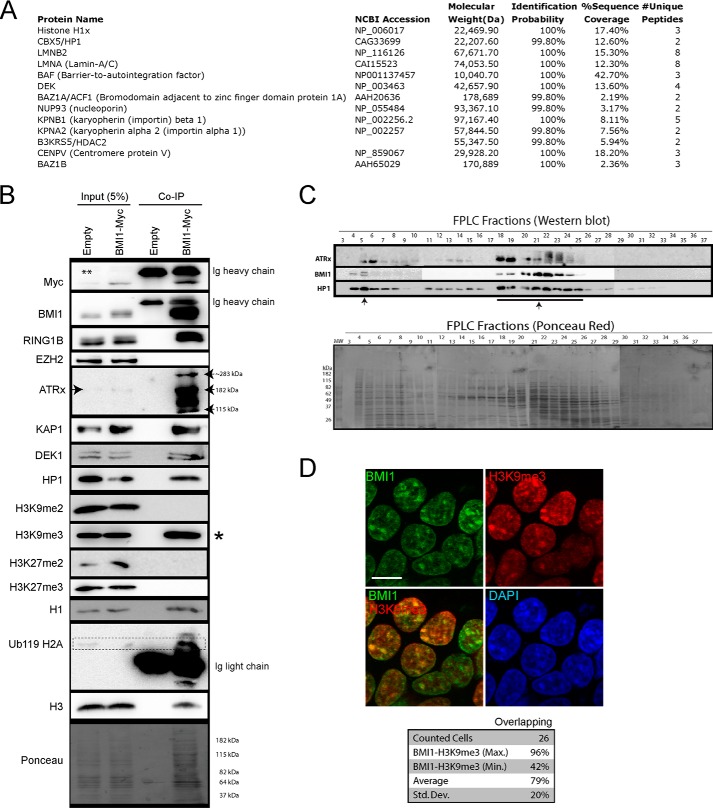
FIGURE 4.
BMI1 co-purifies with architectural heterochromatin proteins. A and B, 293T cells were infected with EFv/CMV-GFP or EFv-BMI1Myc/CMV-GFP viruses. Protein extracts were subjected to IP using an anti-Myc antibody, and immunoprecipitates were resolved by SDS-PAGE and analyzed either by LC-MS/MS (A) or Western blot (B). A, note the co-purification of BMI1 with several heterochromatin proteins and Lamins. B, note the preferential co-purification of BMI1 with histone H3K9me3 (*) and ATRx. The ** symbol on the panel indicates an artifact coming from partial leakage of the second sample. C, native nuclear extracts were size fractionated by FPLC and analyzed by Western blot (upper panel) and Ponceau Red staining (lower panel). Note BMI1 co-fractionation with ATRx and HP1-containg protein complexes (arrows). D, 293T cells were labeled with BMI1 and H3K9me3 antibodies, counterstained with DAPI, and analyzed by confocal microscopy. Note the co-localization of BMI1 with H3K9me3-positive chromatin domains. Scale bar, 10 μm. Quantitative confocal analysis was used to measure the proportion of overlapping signals.