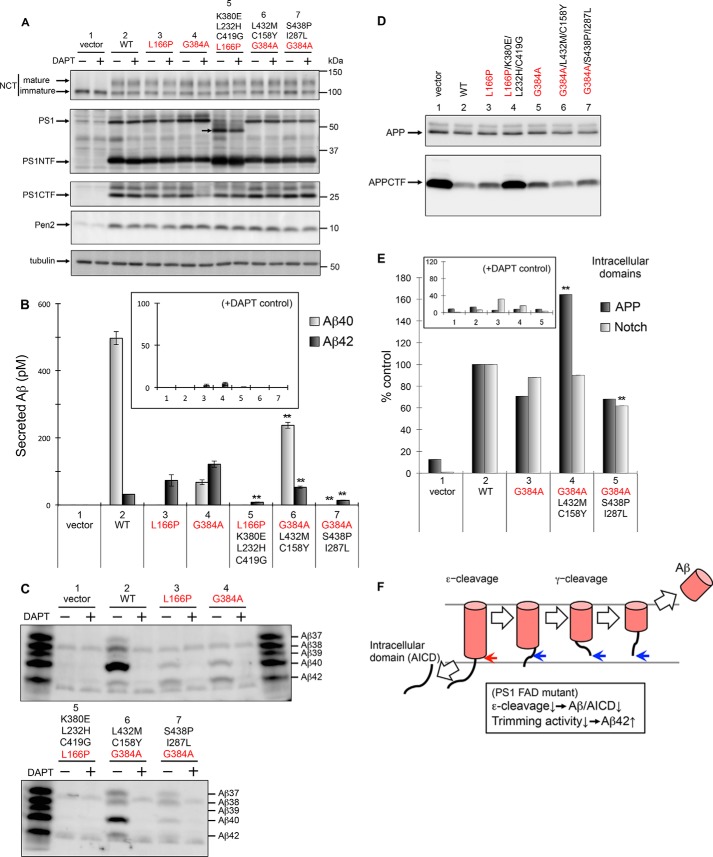
FIGURE 4.
Production of Aβ and the intracellular domains of APP and Notch by PS1 with FAD mutations and secondary suppressors in mouse embryonic fibroblasts. Using the retroviral infection system, PS1/PS2 DKO cells, stably expressing APP-NL, were transfected with WT or mutant PS1, L166P, G384A, L166P/K380E/L232H/C419G, G384A/S438P/I287L, or G384A/L432M/C158Y (A–D). Cells were harvested and analyzed by immunoblotting using antibodies specific to NCT (N1660), PS1 (anti-PS1NT for NTF and G1L3 for CTF), Pen2 (PNT3), or tubulin (DM1A) (A). Secretion of Aβ peptides including Aβ40 and Aβ42 (B and C). After transfection, media were recovered, and the secreted Aβ peptides were quantified by ELISA using antibodies against Aβ40 (gray) or Aβ42 (black) (B). Values for 10 μm DAPT (+), N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester, are shown as the controls (inset) (B). Media were also analyzed using urea/SDS-PAGE followed by immunoblotting with an antibody specific to Aβ (82E1). Synthetic Aβ37, Aβ38, Aβ39, Aβ40, and Aβ42 were loaded as indicated (C). APP and APP CTF in cell lysates were analyzed by immunoblotting (D). Effects of FAD and suppressor mutations on ϵ-cleavage of APP and Notch in a cell-based γ-secretase assay are shown (E). 1210/DKO cells expressing APP and Notch luciferase reporters were infected transiently with recombinant retrovirus encoding PS1 mutants. Relative levels of intracellular domains of APP (black) and Notch (gray) are indicated (n = 3; means ± S.E. of the mean). Values for 10 μm DAPT (+) are shown as a control (inset) (E). Data represent the mean ± S.D.; n = 3. Statistical analyses were performed by one-way analysis of variance followed by Dunnett's multiple comparison test. Asterisks indicate p < 0.01 (**) compared with PS1 L166P or G384A (B and E). FAD and secondary mutations are indicated in red and black, respectively. Schematic model of processive intramembrane cleavage by γ-secretase is shown (F). γ-Secretase cleaves APP in a manner similar to that of endopeptidase (ϵ-cleavage), followed by a mechanism similar to that of carboxypeptidase (γ-cleavage). Decreased total Aβ/APP intracellular domain (AICD) and increased longer Aβ peptides by PS1 FAD mutations can be explained by partial loss of both ϵ- and γ-cleavage activities.