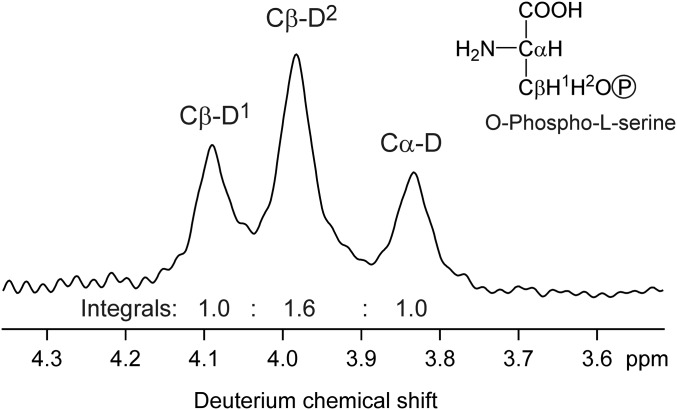
Fig. S2.
Deuterium NMR spectrum of O-Phospho-l-serine (Santa Cruz Biotechnolgy), indicating D isotopomer abundances of serine formed by serine hydroxymethyl transferase during fermentation (33). The integrals of the signals, obtained by deconvolution, are proportional to the abundances of the Cα-D isotopomer and the two D isotopomers at Cβ, labeled Cβ-D1 and Cβ-D2. Although the stereochemical assignments of Cβ-D1 and Cβ-D2 are unknown, their strongly differing integrals show that serine hydroxymethyl transferase creates a strongly uneven D isotopomer ratio at C3 of serine, which, in the photorespiration pathway, is converted into an uneven D isotopomer ratio at C3 of 3-phosphoglycerate.