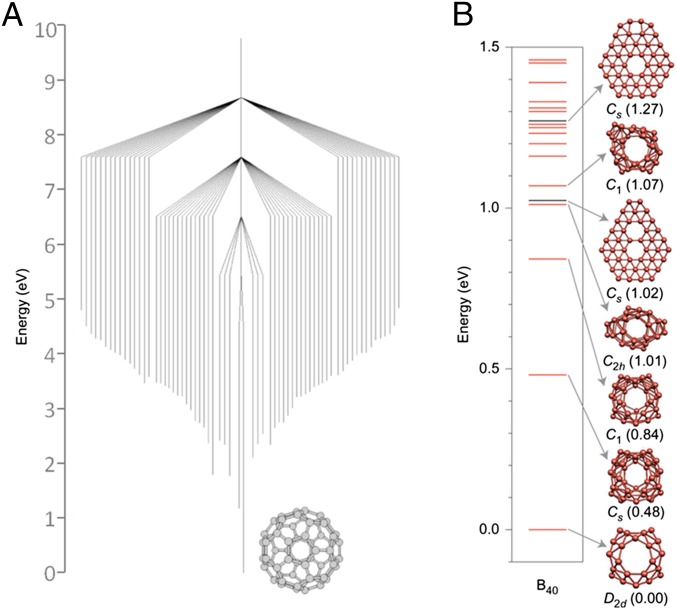
Fig. 1.
(A) “Willow tree” pattern of different C60 isomers, with the lower points of each vertical bar representing the calculated formation enthalpy relative to Ih-C60, and bar heights representing the calculated barrier to transformation. This shows that Ih-C60 is significantly more stable than other isomers and lies at the center of an “energetic funnel.” Adapted from ref. 7, with permission from Macmillan Publishers Ltd.: Nature, copyright 1998. (B) Calculated formation enthalpies of B40, showing the D2d cage structure is significantly more stable than alternative isomers. Reproduced from ref. 12, with permission from Macmillan Publishers: Nature Chemistry, copyright 2014.