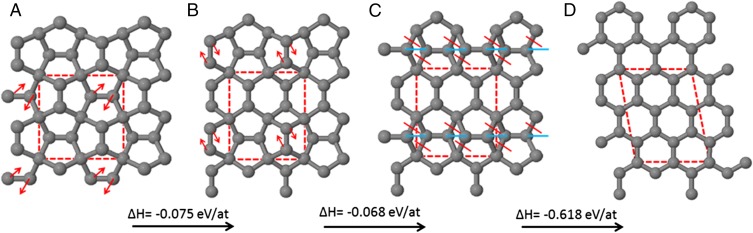
Fig. 2.
A calculated structural transformation route from (A) penta-graphene to (D) graphene; each step is exothermic. Red arrows indicate direction of motion of atoms for 90° rotation of carbon–carbon bonds. Red (blue) lines indicate C–C bonds that are broken (formed). Note that structures A–C were constrained within orthogonal unit cells; this constraint was lifted for step C to D. The final structure, graphene, is 0.761 eV per atom more stable than A. Unit cells are marked with dotted lines; calculated cell dimensions are (A) 5.095 × 5.095 Å, (B) 4.769 × 5.510 Å, (C) 4.888 × 5.318 Å, and (D) 4.883 × 6.476 Å, α = 100.88°.