Abstract
Chronic hepatitis C is both a virologic and a fibrotic disease, with mortality resulting mainly from the complications of cirrhosis and HCC. The aim was to evaluate the impact on of supplementation with a new pharmaceutical complex of silybinvitamin E-phospholipids in patients with chronic hepatitis C treated with Pegylated-Interferon-α2b plus Ribavirin. In this prospective, randomized, placebo controlled, double blind clinical trial, 32 subjects with chronic hepatitis, received Pegylated-Interferon-α2b (1.5 mg/kg per week) plus Ribavirin and placebo, while 32 subjects received the same dosage of Pegylated-Interferon-α2b plus Ribavirin plus association of Silybin 47 mg + vitamin E 15 mg + phospholipids 97 mg in two pill for 12 months. Serum levels of the following markers of liver fibrosis were evaluated: transforming growth factor beta, hyaluronic acid, metalloproteinase 2, amino-terminal pro-peptide of type III procollagen, tissue inhibitor of matrix metalloproteinase type I. The comparison between group A and group B showed a significant difference in ALT (P<0.001), and viremia (P<0.05) after 12 months; in TGF beta levels after 12 months and at follow up (P<0.05); in MMP-2 after 6 months (P<0.05); in PIIINP after 6, 12 months and at follow up (P<0.05); in TIMP-1 after 6, 12 months and at follow up (P<0.001). In conclusion, the supplementation with silybin-vitamin E-phosholipids complex ameliorated the response to Peg-IFN plus RBV treatment and reduced serum levels of markers of liver fibrosis. The ameliorative effect of the complex maybe related to a direct effect on the activation of hepatic stellate cells, or mediated via antioxidants.
Keywords: Silybin, interferons, ribavirin, hepatitis C, liver fibrosis
Introduction
Hepatitis C virus (HCV) was identified in 1989 and is an important cause of parentally transmitted hepatitis [1]. Treatment duration and success rates depend on viral and host factors. The most important factor is viral genotype with genotype 1 or 4 being difficult to treat, and genotype 2 or 3 easier. Other factors important to response are JL 28 B genotype, viral load, presence of fibrosis [2]. The severe results of chronic hepatitis C are cirrhosis, hepatic decompensation and the development of hepatocellular carcinoma, with HCV infection ultimately causing around 350000 deaths per year [3]. Cell death and inflammation constitute two characteristic and linked features of chronic liver disease that promote the development of fibrosis. Fibrogenesis is a multicellular response with hepatic stellate cells, constituting the main effectors-contributing to 90% of extracellular matrix-producing myofibroblasts and inflammatory signalling pathways regulating many of the crucial cell-cell interactions. Diagnosis of hepatic fibrosis represents the wound healing response of the liver to viral, immune and toxic insults [4]. Increased fibrosis stages 3-4 had substantially increased mortality, conversely in the absence of advanced did not have increased overall mortality compared to the reference population [5,6]. Diagnosis of the stage of fibrosis in chronic hepatitis C is essential of making a prognosis and deciding on antiviral therapy. Although hepatic C fibrogenesis has been considered to be irreversible, it is now believed to be a dynamic process with a significant potential for reversal. Administration of Pegylated Interferon-a (Peg-INF-a) and Ribavirin (RBV) may reach eradication rates of 52% in patients with HCV genotype 1 and 80% in patients with other genotypes. Silybin, a flavonolignan, is the lead compound of the extract from Sylibum marianum seeds. Silybin is the component in silymarin that acts as a free radical scavenger, suppressing both the proliferation of hepatic stellate cells and the collagen deposition in vitro in humans [9,10]. Silybin acts both as an antioxidant and as an antifibrotic agent. Silybin also inhibits the production of TNF-alpha, IFN-gamma, IL-2 and IL-4 and increases the expression of IL-10 and i-NOS. Silybin increases the expression of matrix MMPs, reduces the expression of TIMP-1, up-regulates the mRNA expression of TGF-beta and suppresses the proliferation of hepatic stellate cells as an antifibrotic agent. Since activation of hepatic stellate cells is the dominant event in liver fibrosis, most proposed anti-fibrotic therapeutic approaches are based on the molecular mechanisms associated with hepatic stellate cell activation. Silybin is rapidly absorbed when conjugated with a phytosome and vitamin E. The dried S. marianum extract, standardized for its silybin content is approved for the supportive treatment of chronic liver diseases [13]. In this complex, silybin reduces collagen accumulation and lipid peroxidation in rats with induced liver fibrosis, reducing sustained virologic response. The purpose of this study was to prospectively evalutated the effects of supplementation with a new pharmaceutical complex of silybin-vitamin Phospholipids in patients with chronic hepatitis C treated with Peg-IFN-a and RBV. Since fibrosis is common and has adverse effects in all organs, it is an attractive therapeutic target. The efforts to attenuate fibrosis have direct clinical implications. In fact patients with minimal fibrosis have a low risk of development of complications of liver disease during the subsequent two decades; conversely patients with bridging fibrosis or cirrhosis have a higher risk.
Patients and methods
The study was designed as a prospective, randomized, placebo controlled, double-blind clinical trial. The study was conducted at the Department of Senescence, Cannizzaro Hospital, University of Catania (Italy), between June 2010 and December 2012. 70 patients have been enrolled (42 males, 28 females) (Table 1). 64 patients received Peg-IFN-a2b plus ribavirin (group A; n=32) and placebo or Peg-IFN-a 2b plus ribavirin plus association of Silybin 47 mg + vitamin E 15 mg + phospholipids 97 mg in two pills (group B; n=32) for a 12-month period (Figure 1). Patients were randomly divided into 2 groups (Group A and Group B) and stratified according to HCV genotype (1 vs others) and viral load (<600,000 vs >600,000 IU/mL). Patients were randomized into two groups (complex Silybin + vitamin E + phospholipids versus placebo) using permuted-block randomization with an allocation ratio of 1:1 and a block size of 4. Randomization was performed by an independent statistician. Random numbers were assigned to patients according to the sequence of their inclusion and patients received respective study products. Both clinical investigators and patients were blind to the product given. Peg-IFN-a 2b (1.5 mg/kg per week) plus RBV and placebo were administered to subjects in Group A. The dose of RBV was 800mg for body weight less than 60 kg, 1,000 mg between 60 and 75 kg, and 1,200 mg more than 75 kg. Subjects in Group B received Peg-IFN-a 2b and RBV plus complex Silybin 47 mg + vitamin E 15 mg + phospholipids 97 mg administered two times a day per os. Subjects were evaluated before starting therapy, after 6 and 12 months. A follow-up was carried out 6 months after the end of the treatment. Eligible patients were workers who were 18 years of age or older, were infected by HCV and had a quantifiable serum HCV RNA level (as determined by polimerase chain reaction, COBAS AmpliPrep/COBAS TaqMan-ROCHE). HCV infected populations must had elevated serum alanine transaminase levels and findings on liver biopsy consistent with chronic infection. Ineligible patients were those who had other liver diseases, as well as those who were affected by cancer, severe jaundice, pulmonary and renal chronic diseases, prostatic diseases, autoimmune diseases and diabetes mellitus. None of the patients made excessive use of alcohol (>20 g/die) or hepato-toxic drugs. Other causes of exclusion included decompensated cirrhosis, pregnancy, and contraindications for Peg-IFN-a or RBV therapy such as cardiopathy, hemoglobinopathies, hemocromatosis, major depression or other severe psychiatric pathological conditions. Clinical evaluations, hematochemical, virological, instrumental and histological analysis were performed on these patients. All subjects underwent a physical examination and medical interview before treatment. Study recruitment was performed in observation and respect of Helsinki Declaration [14]. All patients gave their informed consent for the study participation and for each invasive procedure they underwent.
Table 1.
Patients characteristics at liver biopsy
| Parameter | Group A n=32 (Peg-IFNa + RBV + placebo) | Group B n=32 (Peg-IFNa + RBV + Silybin-Vit.E-Phospholipids) | p-value |
|---|---|---|---|
| Mean age (years) | 45.2±6.7 | 46.4±6.9 | NS |
| Sex (M/F) | 19/13 | 18/14 | |
| BMI (kg/m2) | 26.2±3.8 | 26.4±3.7 | NS |
| HCV exposure time (years) | 6.8±5.2 | 6.6±5.4 | NS |
| Route of transmission of HCV (no of patients) | |||
| Blood transfusion | 16 (50%) | 15 (46%) | NS |
| Intravenous drug abuse | 5 (15%) | 5 (15%) | NS |
| Occupational | 2 (6%) | 2 (6%) | NS |
| Unknown | 11 (34%) | 10 (31%) | NS |
| HCV genotype | |||
| 1a | 1 (3%) | 1 (3%) | NS |
| 1b | 27 (84%) | 26 (81%) | NS |
| 2a | 2 (6%) | 1 (3%) | NS |
| 3a | 2 (6%) | 4 (12%) | NS |
Figure 1.
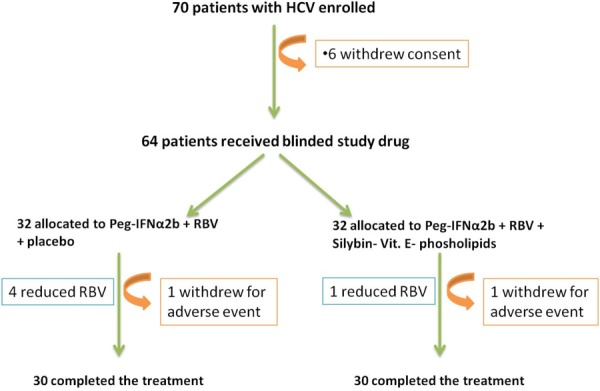
Trial profile of Peg-IFNα2b plus RBV plus complex Silybin, vitamin E and phospholipids.
Serum analysis
All patients underwent a complete virological assay for HBV and HCV. HBsAg (Hepatitis B surface Antigen), anti-HBc IgG (Hepatitis B “core” IgG Antibody), HBeAg (Hepatitis B “e” Antigen), HBeAb (Hepatitis B “e” Antibody), HBV-DNA (Hepatitis B Virus DNA), anti-Delta (Delta virus antibody) assays were performed. We also measured serum HCV RNA concentrations using he COBAS Taq Man HCV RNA assay, version 2.0 (Roche Diagnostics Indianapolis, IN, USA), with a lower limit of quantification of 43 IU/ ml and lower limit of detection of 15 IU/ml. (Anti-HCV antibodies were determined by ELISA (Enzyme-Linked immunosorbent assay E.L.I.S.A. assay-Ortho Diagnostic Systems, Raritan, NJ, USA). Serum samples negative for HCV RNA were re-tested using a more sensitive standardized qualitative PCR assay with a lower limit of detection of about 100 copies/mL) to confirm HCV-RNA disappearance [15]. HCV genotypes and subtypes were identified. HCV viral genotypes were determined by restriction analysis of HCV-RNA 5’UTR [16]. AST and ALT (Aspartate Aminotransferase and Alanine Aminotransferase), γGT (gamma Glutamil Tranferase), total, conjugated and unconjugated bilirubin, serum proteins analysis were performed. We determined plasma levels of transforming growth factor (TGF) beta, hyaluronic acid (HA), and metalloproteinase 2 (MMP-2), amino-terminal pro-peptide of type III procollagen (PIIINP), tissue inhibitor of matrix metalloproteinase type I (TIMP- 1), by ELISA (ELISA human quantikine) which were used as markers of liver fibrosis.
Histological grading assessment
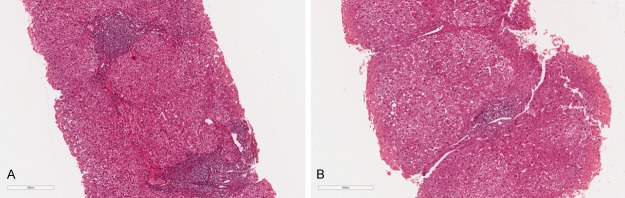
Patients underwent ultrasound-assisted percutaneous biopsy: tissue specimens were obtained with Menghini modified needles (Automatic Aspiration Needle for Liver Biopsy, ACR 16G, 11 cm, manufactured by Sterylab Srl, Milan-Italy). A biopsy was considered adequate for evaluation if the specimen was >1.5 cm long and contained a minimum of 6 portal tracts. Knodell and Ishak Histological activity index (HAI) score was used to assess the histological grading of the disease [17]. The METAVIR scoring system was used to stage liver fibrosis as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and few septa; F3, numerous septa without cirrhosis; F4, cirrhosis [18] Figure 2.
Figure 2.
Representative histological examination. A. Before treatment. hematoxylin-eosin Scheuer score: Portal Activity 3; lobular activity 2; fibrosis 3. B. After treatment hematoxylin-eosin Scheuer score: Portal Activity 2; lobular activity 1; fibrosis 2.
Efficacy and safety assessment
We performed an intention-to-treat (ITT) efficacy analysis. “Sustained virological responders” (SVR) were patients with not identifiable serum HCV RNA at the end of the study. We considered the “relapse” as undetectable HCV-RNA levels at the end of treatment but detectable levels during the follow-up period. Reasons for discontinuation of the treatment were severe adverse events and absence of compliance.
Statistical analysis
Results are expressed as means ± standard deviations. Quantitative data were compared by paired or unpaired Student’s t-test or Mann–Whitney test; the χ-square test was used for analysis of qualitative data. For sample size determination (power =90%, alpha =0.05 a drop-out rate of 20% was assumed and yielded a sample size of 60 patients in total. All results shown in this manuscript were analyzed in the intention-to-treat population. P values <0.05 were considered statistically significant. All statistical analysis were performed using SPSS 15.0 (Chicago, IL).
Results
Demographics characteristics were analogous between the two groups at baseline. The most frequent viral genotype was 1b in Table 1.
Effect of silybin on laboratory parameters
In Group A, we observed a significant decrease in AST (P<0.001) and ALT (P<0.001) and viremia (p<0.05) after 6, 12 months, and at follow up (Table 2). In Group B there was a significant decrease in AST (P<0.001), ALT (P<0.001), and viremia (P<0.001) after 6, 12 months, and at follow up. A significant decrease in HAI score (P<0.05) was observed after 12 months. After 12 months the comparison between group A and group B showed a significant difference in ALT ( 92.4 vs 133.3; P<0.001), in AST 108.2 vs 130 P< 0.01 and viremia (-2.16 versus –3.65; P<0.05). At follow up, we found a significant difference in AST (-90.6 vs 125.0; P<0.05), ALT (-88.8 vs -126; P<0,05), and viremia (-2.39 vs 3.48; P<0.05).
Table 2.
Characteristics of subjects at baseline, after 12 months, and at follow-up
| Group A Peg-IFNa + RBV (n=32) | Group B Peg IFNa + RBV + Silybin-Vit E-Phospholipids (n=32) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Before treatment | After 6 months | After 12 months | Follow-up | Before treatment | After 6 months | After 12 months | Follow-up | |
| AST (IU/l) | 162.4±37.8 | 80.1±37.6C,* | 54.2±32.6C,* | 71.8±29.2C,** | 175.6±41.8 | 70.6±35.2C,* | 44.8±27.1C,* | 50.6±24.2C,** |
| ALT (IU/l) | 161.6±39.6 | 101.2±38.4C,* | 69.2±27.8C,*** | 72.8±24.7C,*** | 170.2±40.6 | 92.8±37.1C,* | 36.9±29.2C,*** | 44.2±28.2C,*** |
| Bilirubin (mmol/l) | 10.9±7.2 | 10.3±6.4A,* | 10.1±6.5A,* | 10.5±6.4A,* | 10.6±7.1 | 10.2±6.3A,* | 10.1±5.9A,* | 10.2±5.6A,* |
| Viremia (106 IU/ml) | 5.40±3.0 | 3.87±2.6B,** | 3.24±2.1B,** | 3.01±2.6B,** | 5.32±2.8 | 2.02±2.4C,** | 1.67±1.8C,** | 1.84±1.3C,** |
| HAI | 10.4±3.2 | - | 8.8±3.4A,* | - | 10.7±3.7 | - | 7.8±3.6B,* | - |
| HA (ng/ml) | 544±210 | 427±284A,* | 402±218B,* | 396±232B,* | 596±232 | 408±201B,* | 396±211B,* | 411±236B,* |
| MMP-2 (ng/ml) | 287±121 | 266±131A,** | 271±134A,* | 278±136A,* | 302±136 | 196±144B,** | 208±167B,* | 206±154B,* |
| TGF-beta (ng/ml) | 51.8±20.2 | 48.2±18.9A,* | 45.2±18.2A,** | 47.1±19.6A,** | 54.2±21.4 | 44.6±20.2A,* | 32.8±18.6C,** | 35.9±19.2B,** |
| PIIINP (ng/ml) | 44.7±6.4 | 41.2±7.8A,** | 39.8±7.6B,** | 40.2±7.8B,** | 43.8±6.9 | 36.2±7.1C,** | 33.4±7.8C,** | 33.8±7.9C,** |
| TIMP-1 (ng/ml) | 487.2±29.6 | 444.2±28.4C,*** | 421±29.8C,*** | 410.4±31.8C | 480.2±31.8 | 396±32.4C,*** | 310.6±31.8C,*** | 334.6±36.2C,*** |
Comparison between groups:
NS;
p<0.05;
p<0.001.
Comparison within groups:
NS;
p<0.05;
p<0.001.
There were no significant differences between groups at baseline.
Effect of silybin on both serum and histological markers of liver fibrosis
In Group A after 12 months significant reduction in HA (P<0.05), PIIINP (P<0.05), and TIMP-1 (P<0.001). At follow up, HA and PPINP levels were still significantly reduced compared to baseline (P<0.05). In Group B there was a significant decrease in PIIINP (P<0.001), and TIMP-1 (P<0.001), HA (P<0.05) and MMP-2 (P<0.05) after 6, 12 months, and at follow up. TGF beta levels were decreased after 12 months (P<0.001) and at follow up (P<0.05). The comparison between group A and group B showed a significant difference in TGF beta levels after 12 months (6.6 vs -21.4; P<0.05) and at follow up (-4.7 vs -18.3; P<0.05); in MMP-2 after 6 months (-21 vs -106; P<0.05); in PIIINP after 6 months (-3.5 vs -7.6; P<0.05), 12 months (-4.9 vs -10.4; P<0.05), and at follow up (-4.5 vs -10; P<0.05); in TIMP-1 after 6 months (-43 vs -84.2; P<0.001), 12 months (-66.2 vs -169.6; P<0.001), and at follow up (-76.8 vs -145.6; P<0.001). We did not observe significant changes in the stages of liver fibrosis according to METAVIR score (Table 3).
Table 3.
Fibrosis score in the subjects included in the study before treatment and at follow up
| Group A Peg-IFN a + RBV (n=32) | Group B Peg IFN a + RBV + Silybin-Vit E-Phospholipids (n=32) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Fibrosis score | Before treatment | Follow-up | P value1 | Before treatment | Follow-up | P value1 | P value2 |
| F0 | 0 (0%) | 1 (3%) | 0.312 | 0 (0%) | 2 (6%) | 0.149 | 0.555 |
| F1 | 6 (18%) | 6 (18%) | 1 | 5 (15%) | 10 (41%) | 0.138 | 0.250 |
| F2 | 8 (25%) | 7 (22%) | 0.764 | 8 (25%) | 5 (15%) | 0.352 | 0.522 |
| F3 | 8 (25%) | 10 (31%) | 0.575 | 10 (31%) | 8 (25%) | 0.575 | 0.575 |
| F4 | 10 (31%) | 8 (25%) | 0.575 | 9 (28%) | 7 (22%) | 0.561 | 0.764 |
P value: comparison within groups;
P value: comparison between groups at follow up.
Discussion
Fibrosis in associated with a number of pathological and biochemical changes leading to structural and metabolic abnormalities as well as with increased hepatic scarring. The progression of liver fibrosis leads to cirrhosis, a condition characterized by distortion of the normal architecture septa and nodule formation, altered blood flow, portal hypertension, hepatocellular carcinoma. In our study we observed a significant decrease of fibrosis markers in HCV patients treated with Peg-IFN plus RBV plus complex Silybin, vitamin E, phospholipids compared to HCV patients treated with Peg-IFN plus RBV alone. In patients inhibition of matrix production has been the primary target of most antifibrotic therapies to date. This has been attempted directly by blocking matrix synthesis and processing or indirectly by inbibiting the activity of TGF-β, the major fibrogenic cytokine. TGF–β is a potent stimulator of the synthesis of extracellular matrix proteins in most fibrogenic cells.
TGF-β antagonists are being tested because neutralizing this potent cytokine would have the dual effect of inhibiting matrix production and accelerating its degradation. Complementary or alternative therapeutic approaches may ameliorate liver damage and reduce side effects and consequently dose reduction in treated patients [19-23]. A sustained virologic response (SVR) is considered the first step towards reducing future HCV mortality. Reduced fibrosis has been reported in HCV patients successfully treated with Pegylated α-interferon and Ribavarin through its effects on viral replication and liver injury [24]. Sustained viral clearance has been fibrosis associated with marked regression of fibrosis, so that long term follow-up of patients cleared of HCV may show more dramatic reversal of disease, than at early time points. Developing antifibrotic treatments against the pathological steps for preventing, delaying and reducing liver fibrosis have become main aims in the treatment of chronic liver disease. Oxidative stress has profound consequences on signal transduction pathways including impaired interferon alpha signalling [25-29]. The antioxidative properties of silybin in chronic hepatitis C may improve the response to interferon in nonresponders to Pegylated-IFN/Ribavirin [27]. The association PegIFN plus Ribavirin with complex Silybin, vitamin E and phospholipids, reduces plasma markers of liver fibrosis. The degree of histological fibrosis and levels of fibrosis markers, may influence the biochemical and viral response in HCV patients. The patients with severe fibrosis (F3-F4) reversal and it is possible that early cirrhosis is easier to reverse than more established cirrhosis. Advances is hepatology have shown that fibrosis and cirrhosis are dynamic, rather than static, process and those are might be reversible. Recently, it has been suggested that cirrhosis occurs in a series of critical steps that if uncontrolled culminates in hepatic deregulation [28-29]. Although some authors hypothesized an antiviral activity against HCV, it is possible that the antioxidant effect could reduce oxidative stress that contributes to fibrosis and carcinogenesis in chronic hepatitis C virus infection [30,33]. Moreover, several recent studies have shown the potential hepato-preventive and therapeutic efficacy of silymarin in different animal models and cell culture systems [34-36]. The silymarine positive effects have been ascribed to the putative antioxidant, anti-inflammatory and anti-proliferative properties based on the modulation of specific signalling pathways of transcription factors and genes expression [37]. Stimulation of fibrogenesis is achieved through a series of cytokines, growth factors, chemokines which activate extracellular matrix producing cells through both paracrine and autocrine loops [38]. The clinical use of silybin, vitamin E and phosholipids complex for treatment of chronic hepatitis C, will depend on future studies addressing the pharmacokinetics, the drug interaction profiles and the optimal dose and dosing of this compound. In our study the silybin, vitamin E and phosholipids complex when added to Peg-IFN plus RBV have shown to ameliorate the response to treatment. The ameliorative effect of the complex can be related to a direct effect on the activation of hepatic stellate cells, or mediated via antioxidants, or through metabolic activity unknown at the moment [37-39]. The treatment if various stages of liver fibrosis and cirrhosis leads to improvements in the impaired liver structure and scarring caused by the conditions. Treatments that could slow the progression of fibrosis may be highly beneficial. While there remain challenges to implementing antifibrotic therapies, with continued progress has come the realistic expectation that fibrosis can be treated, offering hope to million of patients with chronic liver disease worldwide. The improvements of liver fibrosis have been well documented in patients whit hepatitis B and C [40-45].
The fact that several diseases in different organ systems are associated with fibrotic changes suggests not only common pathogenic pathways but also possible similar treatments [46].
The improvement of response to treatment, good compliance of patients and low cost, suggest the need for further studies on the clinical use of this complex.
Acknowledgements
This trial was supported by a grant from the Ministero dell’Università e Ricerca Scientifica e Tecnologica (MIUR).
Disclosure of conflict of interest
None.
Authors’ contribution
All Authors contributed equally to this paper.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 3.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–62. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 4.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 5.Torres DM, Harrison SA. Nonalcoholic fatty liver disease: Fibrosis portends a worse prognosis. Hepatology. 2015;61:1462–4. doi: 10.1002/hep.27680. [DOI] [PubMed] [Google Scholar]
- 6.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for diseasespecific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 7.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Galvano F, Malaguarnera M, Vacante M, Motta M, Russo C, Malaguarnera G, D’Orazio N, Malaguarnera L. The physiopathology of lipoprotein (a) Front Biosci (Schol Ed) 2010;2:866–75. doi: 10.2741/s107. [DOI] [PubMed] [Google Scholar]
- 9.Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–8. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Ann Hepatol. 2009;8:283–91. [PubMed] [Google Scholar]
- 11.Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE HALT-C Trial Group. Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment Pharmacol Ther. 2011;33:127–37. doi: 10.1111/j.1365-2036.2010.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadziyannis SJ, Koskinas JS. Differences in epidemiology, liver disease and treatment response among HCV genotypes. Hepatol Res. 2004;29:129–135. doi: 10.1016/j.hepres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Schümann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J Hepatol. 2003;39:333–40. doi: 10.1016/s0168-8278(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–6. [PubMed] [Google Scholar]
- 15.Stuyver L, Wyseur A, van Arnhem W, Hernandez F, Maertens G. Second-generation line probe assay for hepatitis C virus genotyping. J Clin Microbiol. 1996;34:2259–66. doi: 10.1128/jcm.34.9.2259-2266.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmonds P, Alberti A, Alter HJ, Bonino F, Bradley DW, Brechot C, Brouwer JT, Chan SW, Chayama K, Chen DS, et al. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology. 1994;19:1321–4. [PubMed] [Google Scholar]
- 17.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 18.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 19.Malaguarnera M, Vacante M, Russo C, Gargante MP, Giordano M, Bertino G, Neri S, Malaguarnera M, Galvano F, Li Volti G. Rosuvastatin reduces nonalcoholic fatty liver disease in patients with chronic hepatitis C treated with α-interferon and ribavirin: Rosuvastatin reduces NAFLD in HCV patients. Hepat Mon. 2011;11:92–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Malaguarnera M, Scuderi L, Ardiri A, Malaguarnera G, Bertino N, Ruggeri IM, Greco C, Ozyalcn E, Bertino E, Bertino G. Type II mixed cryoglobulinemia in patients with Hepatitis C Virus: treatment with Pegylated-Inferferon and ribavirin. Acta Medica Mediterranea. 2015;3:651–662. [Google Scholar]
- 21.Malaguarrnera M, Vacante M, Giordano M, Motta M, Bertino G, Pennisi M, Neri S, Malaguarnera M, Li Volti G, Galvano F. L-carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg Interferon α plus ribavirin. World J Gastroenterol. 2011;17:4414–20. doi: 10.3748/wjg.v17.i39.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaguarnera G, Pennisi M, Gagliano C, Vacante M, Malaguarnera M, Salomone S, Drago F, Bertino G, Caraci F, Nunnari G, Malaguarnera M. Acetyl-L-Carnitine Supplementation During HCV Therapy With Pegylated Interferon-α 2b Plus Ribavirin: Effect on Work Performance; A Randomized Clinical Trial. Hepat Mon. 2014;14:e11608. doi: 10.5812/hepatmon.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uccello M, Malaguarnera G, Pelligra EM, Biondi A, Basile F, Motta M. Lipoprotein(a) as a potential marker of residual liver function in hepatocellular carcinoma. Indian J Med Paediatr Oncol. 2011;32:71–5. doi: 10.4103/0971-5851.89775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poynard T. [Do the interferons have an antifibrotic action? The hepatologist’s point of view] . Rev Med Interne. 2002;23(Suppl 4):517s–521s. doi: 10.1016/s0248-8663(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 25.Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest. 2002;22:51–65. [Google Scholar]
- 26.Pietrangelo A, Borella F, Casalgrandi G, Montosi G, Ceccarelli D, Gallesi D, Giovannini F, Gasparetto A, Masini A. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941–9. doi: 10.1016/0016-5085(95)90762-9. [DOI] [PubMed] [Google Scholar]
- 27.Kawada N, Seki S, Kuroki T, Inoue M. Regulation of stellate cell proliferation by lipopolysaccharide: role of endogenous nitric oxide. J Gastroenterol Hepatol. 1998;13(Suppl):S6–13. [PubMed] [Google Scholar]
- 28.Marrazzo G, Bosco P, La Delia F, Scapagnini G, Di Giacomo C, Malaguarnera M, Galvano F, Nicolosi A, Li Volti G. Neuroprotective effect of silibinin in diabetic mice. Neurosci Lett. 2011;504:252–6. doi: 10.1016/j.neulet.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010 Mar 4;362:823–32. doi: 10.1056/NEJMra0901512. doi: 10.1056/NEJMra0901512. Erratum in: N Engl J Med 2011; 364: 490. [DOI] [PubMed] [Google Scholar]
- 30.Sohrabpour AA, Mohamadnejad M, Malekzadeh R. The reversibility of cirrhosis. Aliment Pharmacol Ther. 2012;36:824–32. doi: 10.1111/apt.12044. [DOI] [PubMed] [Google Scholar]
- 31.Tanamly MD, Tadros F, Labeeb S, Makld H, Shehata M, Mikhail N, Abdel-Hamid M, Shehata M, Abu-Baki L, Medhat A, Magder LS, Afdhal NH, Strickland GT. Randomised doubleblinded trial evaluating silymarin for chronic epatiti C in an Egyptian village: study description and 12-month results. Dig Liver Dis. 2004;36:752–9. doi: 10.1016/j.dld.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJ, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275–80. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- 33.Sobesky R, Mathurin P, Charlotte F, Moussalli J, Olivi M, Vidaud M, Ratziu V, Opolon P, Poynard T. Modeling the impact of interferon alfa treatment on liver fibrosis progression in chronic hepatitis C: a dynamic view. The Multivir Group. Gastroenterology. 1999;116:378–86. doi: 10.1016/s0016-5085(99)70135-6. [DOI] [PubMed] [Google Scholar]
- 34.Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schöniger-Hekele M, Holzmann H, Steindl-Munda P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–7. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 35.Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925–36. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 36.Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res. 2005;11:8441–8. doi: 10.1158/1078-0432.CCR-05-1646. [DOI] [PubMed] [Google Scholar]
- 37.Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, Graf TN, Oberlies NH. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci U S A. 2010;107:5995–9. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiff ER. Diagnosing and treating hepatitis C virus infection. Am J Manag Care. 2011;17(Suppl 4):S108–15. [PubMed] [Google Scholar]
- 39.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 40.Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E, Schoonhoven R, Brenner DA, Fried MW. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749–55. doi: 10.1016/s0168-8278(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 41.Arif A, Levine RA, Sanderson SO, Bank L, Velu RP, Shah A, Mahl TC, Gregory DH. Regression of fibrosis in chronic hepatitis C after therapy with interferon and ribavirin. Dig Dis Sci. 2003;48:1425–30. doi: 10.1023/a:1024196201684. [DOI] [PubMed] [Google Scholar]
- 42.Katiyar SK. Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects. Int J Oncol. 2005;26:169–76. [PubMed] [Google Scholar]
- 43.Liu J, Manheimer E, Tsutani K, Gluud C. Medicinal herbs for hepatitis C virus infection: a Cochrane hepatobiliary systematic review of randomized trials. Am J Gastroenterol. 2003;98:538–44. doi: 10.1111/j.1572-0241.2003.07298.x. [DOI] [PubMed] [Google Scholar]
- 44.Jia JD, Bauer M, Cho JJ, Ruehl M, Milani S, Boigk G, Riecken EO, Schuppan D. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen alpha1(I) and TIMP-1. J Hepatol. 2001;35:392–8. doi: 10.1016/s0168-8278(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 45.Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223–9. doi: 10.1097/mog.0b013e3283279668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lampertico M. A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol. 1993;31:456–60. [PubMed] [Google Scholar]