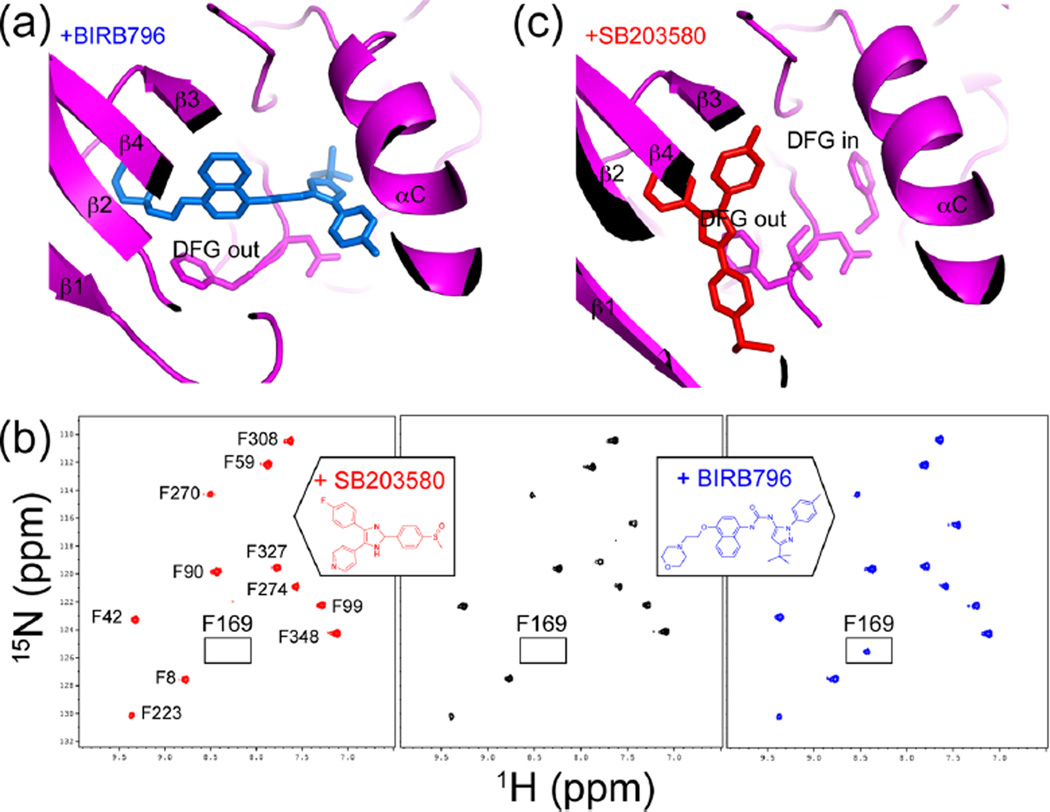
Figure 4.
Conformational selection in binding the p38α inhibitor BIRB796. (a) X-ray structure of p38α (magenta) bound to BIRB796 (blue) (PDB 1KV240) shows selection for the inactive DFG-out conformation. (b) 15N-HSQC spectra show the Phe169 resonance, observed in p38α/BIRB796 (blue) but not observed in apo-p38α (black) or p38α/SB203580 (red) due to line broadening. Line broadening reflects altered dynamics leading to conformational exchange in the intermediate time regime. (c) X-ray structure of p38α (magenta) bound to SB203580 (red) (PDB 2EWA16) shows that both DFG-in and DFG-out conformers can form. Adapted with permission from ref 16. Copyright 2006 John Wiley and Sons.