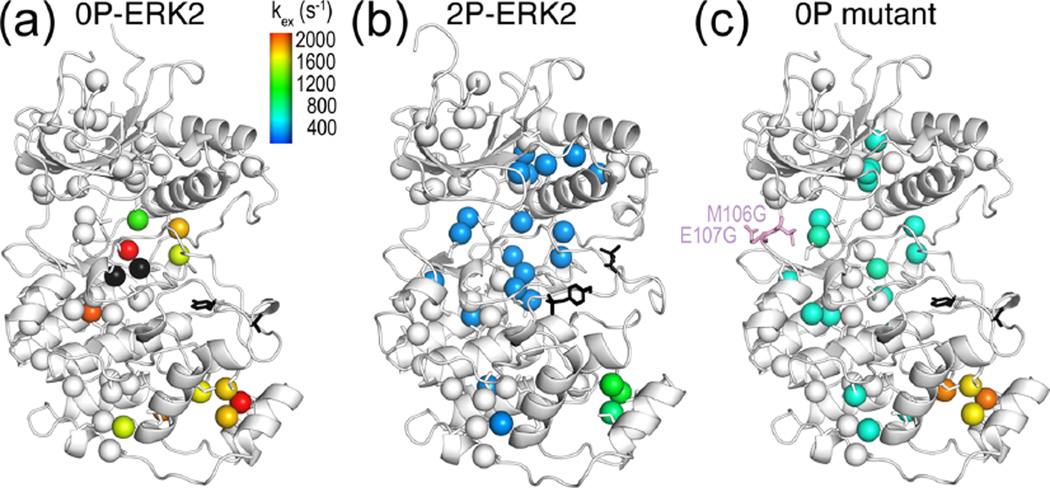
Figure 6.
Global motion accompanies activation of ERK2 by phosphorylation. (a) Isoleucine, leucine, and valine methyls in the kinase core of 0P-ERK2 show large variations in kex, reflecting uncoupled, local motions. Each methyl could be fitted individually, except those in black spheres, which show microsecond to millisecond dynamics but high errors in kex. (b) In contrast, methyls throughout the core of 2P-ERK2 (blue) could be fit together to a two-state exchange process with global kex ≈ 300 s−1. Black sticks show the phosphorylation sites in the activation loop. (c) A mutation at the hinge (M106G, E107G) induces global exchange, even in the absence of phosphorylation. Adapted with permission from ref 20. Copyright 2014 National Academy of Sciences.