Abstract
Maternal infection is an important factor causing neonatal brain injury and later developmental disability. In the present study, we investigated the effects of treadmill exercise intensity on short-term memory, hippocampal neurogenesis, and expression of brain-derived neurotrophic factor (BDNF), and tyrosine kinase receptor B (TrkB) in the rats born of lipopolysaccharide (LPS)-exposed maternal rats. The rats were divided into six groups: control group, mild-intensity exercise group, moderate-intensity exercise group, maternal LPS-exposed group, maternal LPS-exposed and mild-intensity exercise group, maternal LPS-exposed and moderate-intensity exercise group. The rats in the exercise groups were forced to run on a treadmill for 30 min 5 times a week for 4 weeks. The exercise load consisted of running at the speed of 8 m/min for the mild-intensity exercise groups and 14 m/min for moderate-intensity exercise groups. The latency in the step-down avoidance task was deter-mined for the short-term memory. Immunohistochemistry for 5-bro-mo-2′-deoxyuridine was performed to determine hippocampal cell proliferation and neurogenesis. Western blot analysis was performed for the detection of BDNF and TrkB expression. In the present study, tread-mill exercise improved short-term memory deteriorated by maternal LPS exposure. Treadmill exercise increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of the rats born of the LPS-exposed maternal rats. Treadmill exercise increased BDNF and TrkB expression in the hippocampus of the rats born of the LPS-exposed maternal rats. These effects of treadmill exercise were similarly appeared at both mild-intensity and moderate-intensity.
Keywords: Maternal infection, Treadmill exercise, Short-term memory, Neurogenesis, Brain-derived neurotrophic factor, Tyrosine kinase receptor B
INTRODUCTION
Maternal infection is an important factor causing neonatal brain injury and later developmental disability (Back and Rivkees, 2004). Maternal immune response following infection is deleterious to the development of fetus (Rousset et al., 2006), and excessive maternal secretion of cytokines induces fetal inflammatory response syndrome (Bell et al., 2004). Administration of lipopolysaccharide (LPS) to the pregnant rodents elevated proinflammatory cytokines and caused white matter damage of the developing fetal brain (Debillon et al., 2000; Kim et al., 2014).
As the hippocampus is the core center for learning and memory, adult hippocampal neurogenesis is closely associated with hippocampus-dependent learning ability and memory function (Leuner et al., 2006). Physical exercise is known to enhance cell proliferation and/or neurogeneis in the hippocampal dentate gyrus (Kim et al., 2003; Trejo et al., 2001). Several studies have reported that exercise improves cognition, memory capability, and learning ability in rats (Griesbach et al., 2004; Radák et al., 2001). Voluntary exercise following traumatic brain injury facilitates the recovery of cognitive function (Griesbach et al., 2004; Sim, 2014).
Brain-derived neurotrophic factor (BDNF) has been intensively studied and shown to be involved in learning ability, memory function, and synaptic plasticity (Poo, 2001). BDNF has been implicated in the brain plasticity induced by exercise (Cotman and Engesser-Cesar, 2002). In response to BDNF signaling, its specific high-affinity receptor tyrosine kinase receptor B (TrkB) has an enhancing effect on nerve transmission (Kang and Schuman, 1995). The expression of TrkB mRNA has been used as an effective marker for elevated synaptic transmission related to learning and memory (Silhol et al., 2007).
In the present study, we investigated the effects of treadmill exercise intensity on short-term memory, hippocampal neurogenesis, BDNF and TrkB expression in the rats born of LPS-exposed maternal rats. For this study, step-down avoidance task, 5-bromo-2′-deoxyuridine (BrdU) immunohistochemistry, and western blot analysis for BDNF and TrkB were performed.
MATERIALS AND METHODS
Animals and treatments
This study was performed in accordance with the guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. Female Sprague-Dawley rats (180±10 g, 8 weeks old, n=20) were allowed to mate with male rats for 24 hr. The female rats were then individually housed in plastic home cages under the controlled temperature (20ºC±2ºC) and a light–dark cycle consisting of 12 hr of light and 12 hr of darkness (lights on from 7:00 a.m. to 7:00 p.m.). Food and water were made available ad libitum.
The pregnant rats were divided into two groups: the control group and the LPS-exposed group (n=4 in each group). On the 15th, 17th, and 20th day of pregnancy, the pregnant rats in the LPS-exposed group received 1 mL intracervical injections of 0.15 mg/kg LPS (from Escherichia coli, serotype 055:B5, Sigma Chemical Co., St. Louis, MO, USA) suspended in pyrogen-free saline (PFS), and the pregnant rats in the control group were treated with PFS.
After birth, the rat pups were divided into six groups: the control group, the mild-intensity exercise group, the moderate-intensity exercise group, the maternal LPS-exposed group, the maternal LPS-exposed and mild-intensity exercise group, and the maternal LPS-exposed and moderate-intensity exercise group (n=6 in each group).
The rat pups were given intraperitoneal injections of the 50 mg/kg BrdU (Sigma Chemical Co.) dissolved in 0.9% saline, on the exercise day 3, 4, 5 for neurogenesis, and exercise day 18, 19, 20 for cell proliferation.
Exercise protocol
Five weeks after birth, the rats in the exercise groups were forced to run on a motorized treadmill for 30 min 5 times a week for 4 weeks. The exercise load consisted of running at the speed of 8 m/min for the mild-intensity exercise groups and 14 m/min for moderate-intensity exercise groups, at 0º inclination.
Step-down avoidance task
In order to evaluate the short-term memory of the rats, the latency of the step-down avoidance task was conducted as the described previously method (Lee et al., 2006; Park et al., 2014). The rats were trained in a step-down avoidance task. The rats were positioned on a 7×25-cm platform with a height of 2.5 cm, and then allowed to rest on the platform for 1 min. The platform faced a 42×25-cm grid of parallel 0.1-cm-caliber stainless steel bars, which were spaced 1 cm apart. In the training sessions, the animals received a 0.5-mA scramble foot shock for 2 sec immediately upon stepping down. Retention time was assessed on 48 hr after. The interval of rats stepping down and placing all four paws on the grid was defined as the latency of the step-down avoidance task. Any latency over 300 sec was counted as 300 sec.
Tissue preparation
The animals were sacrificed immediately after the completion of the step-down avoidance task. The rats were weighed and given an overdose of Zoletil 50 (10 mg/kg intraperitoneally; Vibac Laboratories, Carros, France). After a complete lack of response was observed, the rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS) and subsequently fixed with freshly prepared 100 mM phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde. The brains were removed and fixed in the same fixative overnight and then transferred to a 30% sucrose solution for cryoprotection. Serial coronal sections of 40-μm thickness were obtained using a freezing microtome (Leica, Nussloch, Germany).
BrdU immunohistochemistry
In order to detect newly generated cells in the hippocampus, BrdU incorporation, which is generally utilized as an indicator for DNA synthesis, was visualized according to a previously described method (Lee et al., 2006; Sim et al., 2014). In brief, the sections were initially permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated with 50% formamide-2×standard saline citrate at 65ºC for 2 hr, denaturated in 2 N HCl at 37ºC for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Afterwards, the sections were incubated overnight at 4ºC with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed 3 times with PBS and incubated for 1 hr with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were then incubated for another 1 hr with avidin-peroxidase complex (1:100; Vector Laboratories). For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.03% H2O2, 0.02% 3,3′-diaminobenzidine (DAB) and 40 mg/mL nickel chloride (nickel-DAB) for 5 min.
After BrdU-specific staining, we performed counter-staining on the same sections using a mouse anti-neuronal nuclei (NeuN) antibody (1:300; Chemicon International, Temecula, CA, USA). The sections were then washed 3 times with PBS, incubated for 1 hr with a biotinylated antimouse secondary antibody, and processed with the VECTASTAIN ABC Kit (1:100; Vector Laboratories). For staining, the sections were allowed to react with 0.02% DAB and 0.03% H2O2 in 50 mM Tris–HCl (pH 7.6) for 5 min and the sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted with Permount (Fisher Scientific, Fair Lawn, NJ, USA).
Western blot analysis
Western blot for BDNF and TrkB was performed according to a previously described method (Heo et al., 2014; Kim et al., 2005). Protein extracts from brain tissue were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein separation was performed using a 10% polyacrylamide with 0.05% bis-acrylamide according to published procedures. Proteins were then transferred to nitrocellulose and the blots were probed with anti-BDNF (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-TrkB (1:1,000; Santa Cruz Biotechnology). Peroxidase anti-rabbit IgG (1:2,000; Vector Laboratories) was used as a secondary antibody. Immunoreactivity was detected by enhanced chemiluminescence (Santa Cruz Biotechnology). Film autoradiograms were exposed from 5 to 15 min.
Data analysis
Statistically significant differences were determined by one-way analysis of variance followed by Duncan post hoc analysis. The results were expressed as the mean±standard error of the mean. Differences were considered significant at P<0.05.
RESULTS
Effect of treadmill exercise on the short-term memory
Latency in the step-down avoidance task was decreased in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity and moderate-intensity increased latency in the step-down avoidance task in the rats born of the LPS-exposed maternal rats. Treadmill exercise exerted no significant effect on the latency in the rats born of the normal maternal rats (Fig. 1).
Fig. 1.
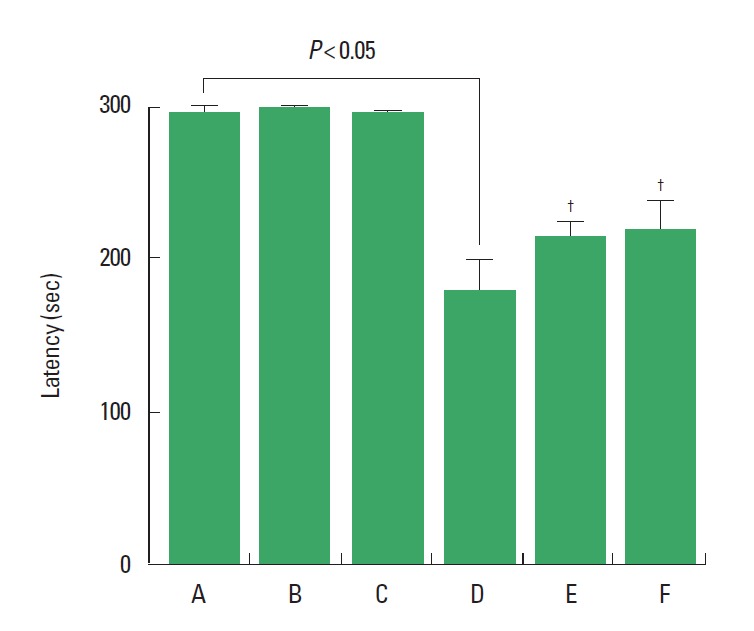
Effect of treadmill exercise on short-term memory in the step-down avoidance task. (A) Control group, (B) mild-intensity exercise group, (C) moderate-intensity exercise group, (D) maternal lipopolysaccharide (LPS)-exposed group, (E) maternal LPS-exposed and mild-intensity exercise group, (F) maternal LPS-exposed and moderate-intensity exercise group. The data are presented as the mean±standard error of the mean. †P<0.05 compared to the maternal LPS-exposed group.
Effect of treadmill exercise on the cell proliferation and cell survival in the hippocampal dentate gyrus
Fig. 2 shows BrdU-positive cells in the subgranular layer (A) and in the granular cell layer (B) of the hippocampal dentate gyrus. BrdU-positive cells in the subgranular layer of the hippocampal dentate gyrus represent cell proliferation. BrdU-positive and NeuN-positive cells in the granular cell layer of the hippocampal dentate gyrus represent neurogenesis.
Fig. 2.
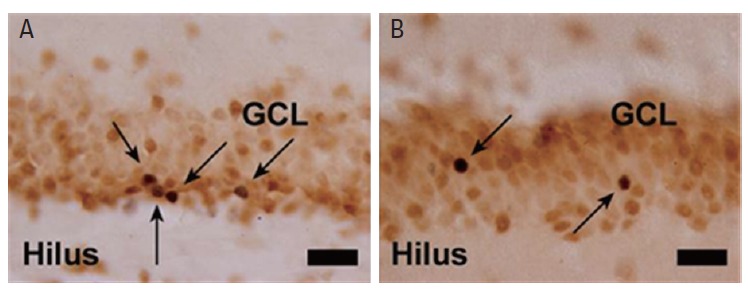
Photomicrographs of 5-bromo-2′-deoxyuridine (BrdU)-positive cells. (A) BrdU-positive cells in the subgranular zone of the hippocampal dentate gyrus (black arrows). (B) BrdU-positive cells in the granular cell layer of the hippocampal dentate gyrus (black arrows). The scale bar represents 25 μm. GCL, granular cell layer.
Cell proliferation in the subgranular zone of the hippocampal dentate gyrus was decreased in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity and moderate-intensity enhanced cell proliferation in the subgranular zone of the hippocampal dentate gyrus in the rats born of the LPS-exposed maternal rats. Treadmill exercise at moderate-intensity enhanced cell proliferation in the subgranular zone of the hippocampal dentate gyrus in the rats born of the normal maternal rats (Fig. 3).
Fig. 3.
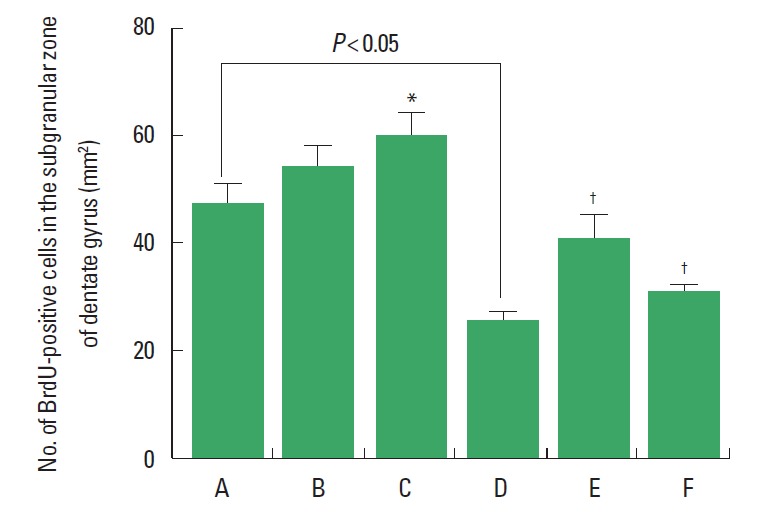
Effect of treadmill exercise on cell proliferation in the subgranular zone of the hippocampal dentate gyrus. (A) Control group, (B) mild-intensity exercise group, (C) moderate-intensity exercise group, (D) maternal lipopolysaccharide (LPS)-exposed group, (E) maternal LPS-exposed and mild-intensity exercise group, (F) maternal LPS-exposed and moderate-intensity exercise group. Data are presented as the mean±standard error of the mean. *P<0.05 compared to the control group. †P<0.05 compared to the maternal LPS-exposed group.
Neurogenesis in the granular cell layer of the hippocampal dentate gyrus was decreased in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity and moderate-intensity enhanced neurogenesis in the granular cell layer of the hippocampal dentate gyrus in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity and moderate-intensity enhanced neurogenesis in the granular cell layer of the hippocampal dentate gyrus in the rats born of the normal maternal rats (Fig. 4).
Fig. 4.
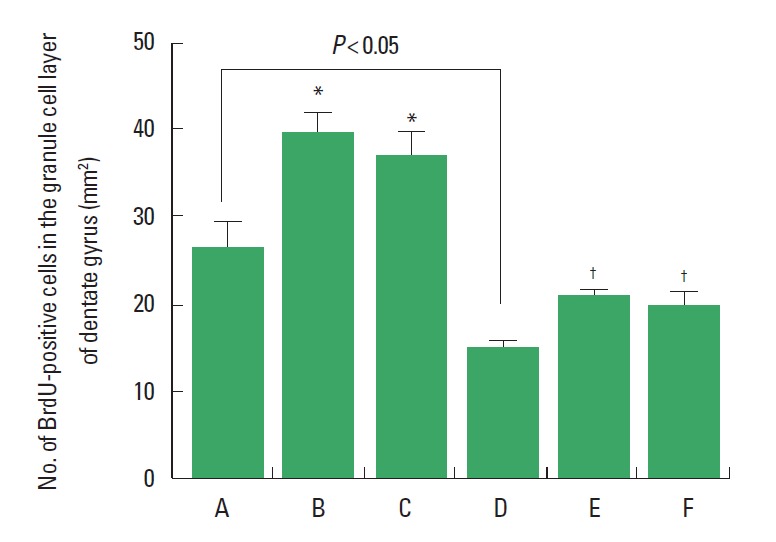
Effect of treadmill exercise on neurogenesis in the granular cell layer of the hippocampal dentate gyrus. (A) Control group, (B) mild-intensity exercise group, (C) moderate-intensity exercise group, (D) maternal lipopolysaccharide (LPS)-exposed group, (E) maternal LPS-exposed and mild-intensity exercise group, (F) maternal LPS-exposed and moderate-intensity exercise group. Data are presented as the mean±standard error of the mean. *P<0.05 compared to the control group. †P<0.05 compared to the maternal LPS-exposed group.
Effect of treadmill exercise on the expressions of BDNF and TrkB in the hippocampus
BDNF expression in the hippocampus was decreased in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity increased BDNF expression in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity suppressed BDNF expression in the rats born of the normal maternal rats, in contrast, treadmill exercise at moderate-intensity enhanced BDNF expression in the rats born of the normal maternal rats (Fig. 5).
Fig. 5.
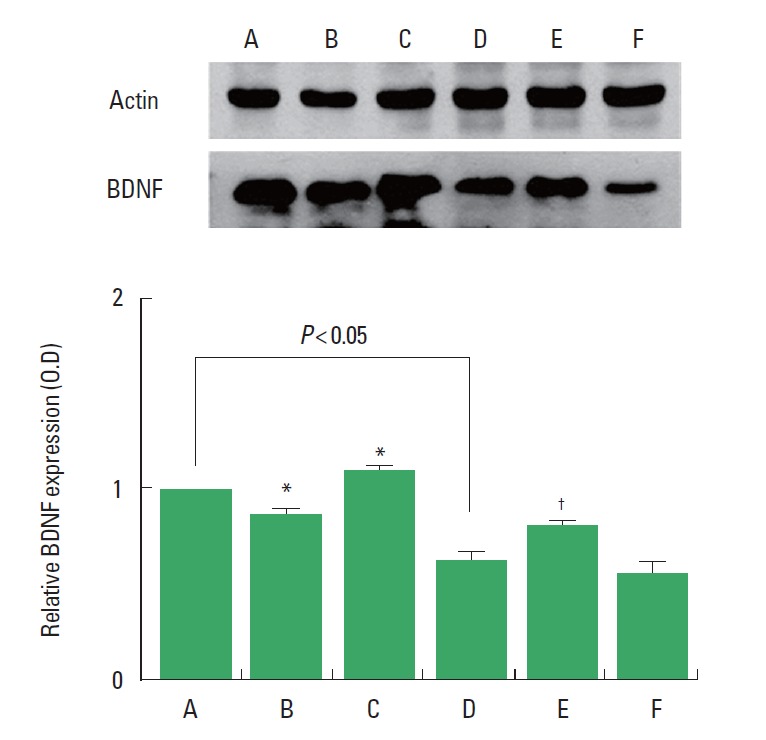
Effect of treadmill exercise on brain-derived neurotrophic factor (BDNF) expression in the hippocampus. (A) Control group, (B) mild-intensity exercise group, (C) moderate-intensity exercise group, (D) maternal lipopolysaccharide (LPS)-exposed group, (E) maternal LPS-exposed and mild-intensity exercise group, (F) maternal LPS-exposed and moderate-intensity exercise group. The data are presented as the mean±standard error of the mean. *P<0.05 compared to the control group. †P<0.05 compared to the maternal LPS-exposed group.
TrkB expression in the hippocampus was decreased in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity and moderate-intensity increased TrkB expression in the rats born of the LPS-exposed maternal rats. Treadmill exercise at mild-intensity and moderate-intensity enhanced TrkB expression in the rats born of the normal maternal rats (Fig. 6).
Fig. 6.
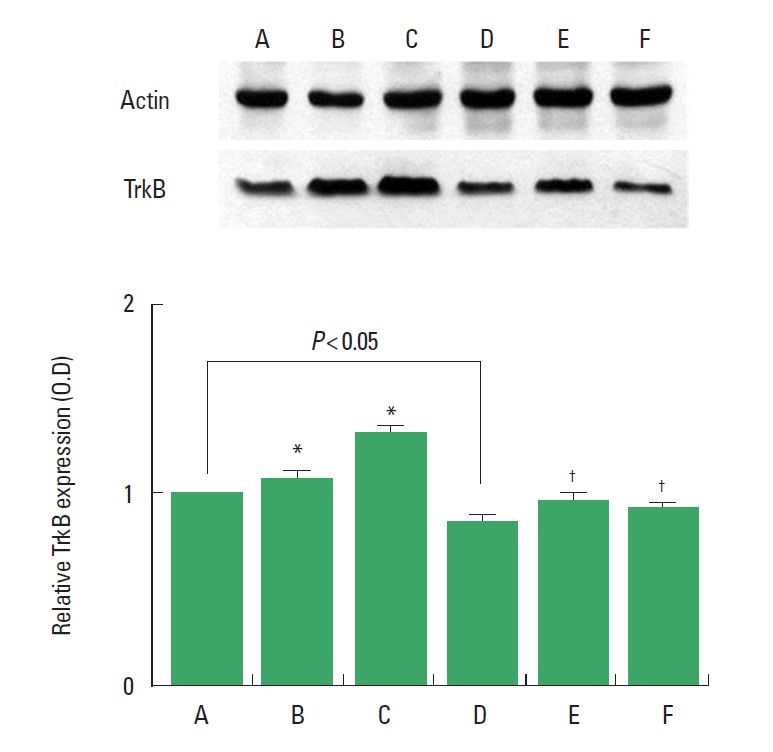
Effect of treadmill exercise on tyrosine kinase receptor B (TrkB) expression in the hippocampus. (A) Control group, (B) mild-intensity exercise group, (C) moderate-intensity exercise group, (D) maternal LPS-exposed group, (E) maternal LPS-exposed and mild-intensity exercise group, (F) maternal LPS-exposed and moderate-intensity exercise group. The data are presented as the mean±standard error of the mean. *P<0.05 compared to the control group. †P<0.05 compared to the maternal LPS-exposed group.
DISCUSSION
In animal studies, exercise increases neuronal activity in the brain (Kim et al., 2005), and exercise might act as a gate that primes the brain to respond to environmental stimulation, while simultaneously increasing the ability of neurons to resist insult (Cotman and Berchtold, 2002). The present results demonstrated that treadmill exercise in both mild- and moderate-intensity enhanced short-term memory in the rats born of the LPS-exposed maternal rats.
The hippocampus is a brain region that retains the ability to produce new neurons in adulthood (Pawluski and Galea, 2007). Progenitor cells reside in the subgranular zone of the dentate gyrus and newly produced daughter cells can migrate into the granule cell layer and make appropriate connections with CA3 pyramidal cells (Zhao et al., 2006). Adult neurogenesis is composed of at least two components: cell proliferation and cell survival (Pawluski and Galea, 2007). Cell proliferation refers to the production of new cells, whereas cell survival refers to the number of new cells that survive to maturity. Factors affecting cell proliferation may modulate mitosis of progenitor cells (Ormerod and Galea, 2001) and factors affecting cell survival may influence differentiation and maturation of cells into mature neurons (Ormerod and Galea, 2001). The number of new neurons is increased by enhancing cell proliferation and by enhancing survival of new neurons. Therefore, it is important to understand how both the proliferation and the survival of new neurons are regulated. In the adult rodents, it is well documented that exercise enhances cell proliferation and/or neurogenesis in the hippocampus (Kim et al., 2003; Trejo et al., 2001). Exercise-induced hippocampal neurogenesis has been suggested to enhance learning ability and memory capability (Sim et al., 2014; Snyder et al., 2005).
Cell proliferation and cell survival in the hippocampus were examined separately in this study. The rats born of the LPS-exposed maternal rats showed lower level of cell proliferation in the subgranular zone compared with the normal rats. However, treadmill exercise increased cell proliferation in the subgranular zone of hippocampal dentate gyrus. Furthermore the rats born of the LPS-exposed maternal rats showed fewer surviving new neurons in the granule cell layer compared with the normal rats. However, treadmill exercise also increased neurogenesis in the granule cell layer of hippocampal dentate gyrus. Our present results showed that treadmill exercise in both mild-intensity and moderate-intensity increased cell proliferation and neurogenesis in the hippocampus of rats born of the LPS-exposed maternal rats.
Hippocampal BDNF is known to be increased during learning tasks (Hall et al., 2000; Mizuno et al., 2000). BDNF expression induced by exercise increases neurogenesis and enhances long-term potentiation of the hippocampus (Farmer et al., 2004). Hippocampal BDNF mediates the ability of exercise to enhance learning and memory (Heo et al., 2014; Vaynman et al., 2004). In the present results, BDNF and TrkB expression in the hippocampus was decreased in the rats born of the LPS-exposed maternal rats, however, moderate-intensity treadmill exercise increased BDNF and TrkB expression in the rats born of the LPS-exposed maternal rats.
In the present study, treadmill exercise improved short-term memory deteriorated by maternal LPS exposure. The improving effect of treadmill exercise can be ascribed to the enhancing effect of treadmill exercise on cell proliferation and neurogenesis. These effects of treadmill exercise were similarly appeared at mild-intensity and moderate-intensity. The present study suggests the possibility that treadmill exercise in early life might facilitate recovery from maternal infection-induced brain damage.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2011-356-G00013).
Footnotes
CONFLIC OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Hallenbeck JM, Gallo V. Determining the fetal inflammatory response in an experimental model of intrauterine inflammation in rats. Pediatr Res. 2004;56:541–546. doi: 10.1203/01.PDR.0000139407.89883.6B. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30:75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Debillon T, Gras-Leguen C, Vérielle V, Winer N, Caillon J, Rozé JC, Gressens P. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res. 2000;47:736–742. doi: 10.1203/00006450-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Heo YM, Shin MS, Kim SH, Kim TW, Baek SB, Baek SS. Treadmill exercise ameliorates disturbance of spatial learning ability in scopolamine-induced amnesia rats. J Exerc Rehabil. 2014;10:155–161. doi: 10.12965/jer.140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kim K, Shin MS, Cho HS, Kim YP. Effects of endurance exercise on expressions of glial fibrillary acidic protein and myelin basic protein in developing rats with maternal infection-induced cerebral palsy. J Exerc Rehabil. 2014;10:9–14. doi: 10.12965/jer.140084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res. 2005;1052:16–21. doi: 10.1016/j.brainres.2005.05.070. [DOI] [PubMed] [Google Scholar]
- Kim YP, Kim HB, Jang MH, Lim BV, Kim YJ, Kim H, Kim SS, Kim EH, Kim CJ. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. 2003;24:114–117. doi: 10.1055/s-2003-38202. [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim H, Lee JW, Kim YS, Yang HY, Chang HK, Lee TH, Shin MC, Lee MH, Shin MS, Park S, Baek S, Kim CJ. Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain Dev. 2006;28:147–154. doi: 10.1016/j.braindev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Park MS, Oh HA, Ko IG, Kim SE, Kim SH, Kim CJ, Kim HB, Kim H. Influence of mild traumatic brain injury during pediatric stage on short-term memory and hippocampal apoptosis in adult rats. J Exerc Rehabil. 2014;10:148–154. doi: 10.12965/jer.140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Radák Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvári M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Rousset CI, Chalon S, Cantagrel S, Bodard S, Andres C, Gressens P, Saliba E. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–433. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- Silhol M, Arancibia S, Maurice T, Tapia-Arancibia L. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience. 2007;146:962–973. doi: 10.1016/j.neuroscience.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Sim YJ. Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer’s disease rats. J Exerc Rehabil. 2014;10:81–88. doi: 10.12965/jer.140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


