Abstract
Depression is a major cause of disability and one of the most common public health problems. In the present study, antidepressive effect of treadmill exercise on chronic mild stress (CMS)-induced depression in rats was investigated. For this, sucrose intake test, immunohistochemistry for 5-bromo-2′-deoxyuridine, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling staining, and Western blot analysis for brain-derived neurotrophic factor, cyclic adenosine monophosphate response element binding protein, and endothelial nitric oxide synthase were conducted. Following adaptation to the animal vivarium and two baseline fluid intake tests, the animals were divided into four groups: the control group, the CMS-induced depression group, the CMS-induced depression and exercise group, and the CMS-induced depression and fluoxetine-treated group. The animals in the CMS groups were exposed to the CMS conditions for 8 weeks and those in the control group were exposed to the control conditions for 8 weeks. After 4 weeks of CMS, the rats in the CMS-induced depression and exercise group were made to run on a motorized treadmill for 30 min once a day for 4 weeks. In the present results, treadmill exercise alleviated CMS-induced depressive symptoms. Treadmill exercise restored sucrose consumption, increased cell proliferation, and decreased apoptotic cell death. The present results suggest the possibility that exercise may improve symptoms of depression.
Keywords: Depression, Treadmill exercise, Chronic mild stress, Fluoxe-tine
INTRODUCTION
Depression is a major cause of disability and one of the most common public health problems, with 10% to 20% lifetime prevalence in worldwide. Antidepressants, such as tricyclic antidepressants and monoamine oxidase inhibitors, are mainly attributable to the modulation of noradrenergic and serotonergic functions (Delgado, 2000). Unfortunately, these drugs have many adverse effects as a result of direct or indirect interactions with multiple receptors (Kent et al., 2000). Selective serotonin reuptake inhibitors lead to the indiscriminate activation of all serotonin (5-hydroxytryptamine) receptors, and are consequently associated with a number of adverse effects (Stahl, 1998).
Neuronal plasticity or remodeling is closely associated with cellular and behavioral mechanisms of learning and memory process (Duman, 2002). Neuronal plasticity is absolutely necessary for adequate functioning of an individual in the continuously changing environment (Fuchs et al., 2004). In the prefrontal and cingulated cortex, metabolism and volume are reduced in depression patients (Manji et al., 2001; Manji and Duman, 2001). Postmortem morphometric brain studies in mood disorders demonstrated cellular atrophy and/or loss (Manji et al., 2003). One of the most consistent effects of stress is atrophy of hippocampal neurons, and depression also induces hippocampal atrophy (Sheline et al., 2003). Major depressive disorders may be associated with an impairment of structural plasticity and cellular resilience, and antidepressants act by normalizing these impairments (Manji and Duman, 2001).
Molecular elements regulating neuronal plasticity are also involved in the actions of antidepressants. These elements include up-regulation of transcription factors, such as the cyclic adenosine monophosphate response element binding protein (CREB) and brain-derived neurotrophic factor (BDNF) (Duman, 2002). Antidepressants exert major effects through signaling pathways of neuroplasticity and cell survival (D’Sa and Duman, 2002; Manji et al. 2001). Voluntary physical activity enhanced BDNF transcription in several hippocampal areas, both on its own and in combination with anti-depressants (Russo-Neustadt et al., 2000).
Endothelial nitric oxide synthase (eNOS) is a downstream mediator for vascular endothelial growth factor and angiogenesis. eNOS regulates BDNF expression in the ischemic brain disease and influences progenitor cell proliferation, neuronal migration, and neurite outgrowth and eNOS affects functional recovery after stroke (Chen et al., 2005). The levels of both plasma NOx and platelet eNOS activity were significantly lower in the subjects with major depression compared with the healthy control subjects (Chrapko et al., 2004).
In the present study, antidepressive effect of treadmill exercise on chronic mild stress (CMS)-induced depression in rats was investigated. For this, sucrose intake test, immunohistochemistry for 5-bromo-2′-deoxyuridine (BrdU), terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining, and western blot analysis for BDNF, CREB, and eNOS were conducted.
MATERIALS AND METHODS
Animals and treatments
Male Sprague-Dawley rats weighing 100±10 g (4 weeks of age) were used for the experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The animals were housed under laboratory conditions at a controlled temperature (20ºC±2ºC) and maintained under light-dark cycles, each consisting of 12 hr of light and 12 hr of darkness (lighting from 7:00 a.m. to 7:00 p.m.) with food and water made available ad libitum. Sucrose solution (1%) was available ad libitum for 1 week preceding the experimental procedures to allow for adaptation to the taste of the sucrose (Grippo et al., 2005). Following adaptation to the animal vivarium and two baseline fluid intake tests, the animals were divided into four groups (n=12 in each group): the control group, the CMS-induced depression group, the CMS-induced depression and exercise group, and the CMS-induced depression and fluoxetine (Eli Lilly and Company, Indianapolis, IN, USA)-treated group. The animals in the CMS groups were exposed to the CMS conditions for 8 weeks and those in the control group were exposed to the control conditions for 8 weeks. Sucrose intake tests were conducted weekly during the CMS period (Grippo et al., 2005). After 4 weeks of CMS, each animal was injected intraperitoneally with BrdU (50 mg/kg; Sigma Chemical Co., St. Louis, MO, USA) for 4 weeks (5 times per a week).
Sucrose intake test
Sucrose intake test was employed to assess anhedonia. Anhedonia is defined as a reduction in sucrose intake and sucrose preference relative to the control group and baseline value. Sucrose intake test consisted of first removing the food and water from each rat’s cage for a period of 20 hr. All animals (both CMS group and control group) were deprived of food and water prior to the sucrose intake test. Water and 1% sucrose were then placed on the cages in preweighed plastic bottles, and the animals were allowed to consume the fluids for a period of 1 hr. The bottles were then removed and weighed. Two baseline fluid intake tests were performed, separated during 5 days, and the results were averaged. Sucrose intake tests were conducted weekly throughout the CMS period. Sucrose intake was calculated on an absolute basis (sucrose and water intake separately) similar to previous studies with the CMS protocol (Grippo et al., 2005).
Chronic mild stress
Following two baseline fluid intake tests, the animals were randomly separated into four groups. The modified CMS procedure employed method described elsewhere (Grippo et al., 2005), and this procedure was designed to minimize pain and discomfort while maximizing the unpredictable nature of the stressors (Table 1). Briefly, the rats in the CMS groups were exposed to the following stressors in random order: continuous overnight illumination (two 12-hr periods), 40º cage tilt along the vertical axis (one 6-hr period), paired housing (one 16-hr period and one 4-hr period), damp bedding (300-mL water spilled into bedding; one 16-hr period), water deprivation (one 16-hr period) exposure to an empty water bottle immediately following the 16-hr period of acute water deprivation (one 1-hr period), and white noise (−90 dB; one 4-hr period of continuous noise and one 3-hr period of continuous noise). Table 1 shows the timing and length of all stressors used in the CMS procedure. The stressors were presented randomly during 1 week, and then repeated during 8 weeks. Control animals were left undisturbed in their home cages throughout the 8-week period with the exception of general handling (i.e., regular cage cleaning and measuring body weight), which was matched to that of the CMS groups. All rats were decapitated 5 days after finishing CMS procedure.
Table 1.
The timing and length of all stressors used in the chronic mild stress procedure
| Stressor | Sunday | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|---|---|---|---|---|---|---|
| Food deprivation | 16:00→ | 12:00 | |||||
| Water deprivation | 16:00→ | 12:00 | 18:00→ | 10:00 | |||
| Continuous lighting | 19:00→ | 7:00 | 19:00→ | 7:00 | |||
| Cage tilt | 10:00→16:00 | ||||||
| Paired housing | 18:00→ | 10:00 | 18:00→22:00 | ||||
| Damp bedding | 22:00→ | 14:00 | |||||
| Empty water bottle | 10:00→11:00 | ||||||
| White noise | 13:00→16:00 | 11:00→15:00 | |||||
| Fluid intake test | 12:00→13:00 |
Treadmill exercise protocol
After 4 weeks of CMS, the rats in the CMS-induced depression and exercise group were made to run on a motorized treadmill for 30 min once a day for 4 weeks (5 times per a week). The exercise regimen consisted of running at 3 m/min for the first 5 min, 5 m/min for the next 5 min, and then 8 m/min for the last 20 min with 0% grade. The animals in the other groups remained on treadmill without running for 30 min (Lee et al., 2003).
Fluoxetine treatment
After 4 weeks of CMS, the rats in the CMS-induced depression and fluoxetine-treated group received 10-mg/kg fluoxetine (Eli Lilly and Company) orally, and those in the other groups received equivalent amount of water orally once a day for 4 weeks (5 times per a week).
Tissue preparation
The animals were first fully anesthetized with Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and then fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40-μm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
BrdU immunohistochemistry
BrdU immunohistochemistry was used for the detection of newly generated cells in the dentate gyrus, as a previously described method (Jang et al., 2002; Sim, 2014). The sections were first permeabilized by incubating in 0.5% Triton X-100 in PBS for 20 min. They were then incubated in 50% formamide-2 × standard saline citrate at 65ºC for 2 hr, denaturated in 2 N HCl at 37ºC for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Afterwards, the sections were incubated overnight at 4ºC with a BrdU-specific mouse monoclonal antibody (1:600; Boehringer Mannheim, Mannheim, Germany). The sections were then washed three times with PBS and incubated for 1 hr with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA. USA). The sections were then incubated for another 1 hr with VECTASTAIN Elite ABC Kit (1:100; Vector Laboratories). For staining, the sections were incubated in a reaction mixture consisting of 0.02% 3,3′-diaminobenzidine containing nickel chloride (40 mg/mL; nickel-DAB) and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.6) for 5 min. The sections were then washed three times with PBS and mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount (Fisher Scientific, Fair Lawn, NJ, USA).
TUNEL staining
For visualization of apoptotic cell death, TUNEL staining was performed using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) as previously described (Heo et al., 2014; Jang et al., 2002). Briefly, sections were post-fixed in ethanol-acetic acid (2:1) and rinsed. Then, the sections were incubated with proteinase K (100 μg/mL), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using converter-POD with nickel-DAB. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount.
Western blot
Western blot for BDNF, CREB, and eNOS was performed according to the previously described method (Heo et al., 2014; Kim et al., 2015). The hippocampus was removed from the rat brain, and trimmed off onto a chilled surface. Following tissue homogenization with ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% deoxycholic acid, 1% NP40, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, the samples were centrifuged at 3,000 × g for 15 min at −4ºC. The supernatant fraction was collected, and the protein concentration determined by Bradford assay. Thirty micrograms of total protein were electrophoresed on the SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane (Schleicher & Schuell GmbH, Dassel, Germany). Mouse antiactin antibody, rabbit anti-BDNF antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-CREB antibody (1:1,000; Upstate, Lake Placid, NY, USA), and mouse anti-eNOS antibody (1:1,000; BD Sciences, Franklin Lakes, NJ, USA) were used as the primary antibodies. Horseradish peroxidase-conjugated antimouse antibody (1:1,000; Santa Cruz Biotechnology) for actin, eNOS, and antirabbit antibody (1:1,000; Santa Cruz Biotechnology) for BDNF, CREB were used as the secondary antibodies. Band detection was performed using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech GmbH, Freiburg, Germany).
Data analysis
Images were captured with video camera attached to light microscope (Olympus, Tokyo, Japan) and data were analyzed using Image-Pro Plus software (Media Cybernetics Inc., Silver Spring, MD, USA). The numbers of BrdU-positive and TUNEL-positive cells in the dentate gyrus were counted hemilaterally using the Image-Pro Plus software and expressed as the number of cells per square millimeter (mm2) of the granular layer. In the western blotting, the mean optical density for each group was measured using the Image-Pro Plus software and expressed as relative intensity where control group was assigned as 1.
Statistical analysis was performed using one-way analysis of variance followed by Duncan post hoc test. The results are presented as the mean±standard error of the mean. Differences were considered significant at P<0.05.
RESULTS
Sucrose intake test
Sucrose intake was decreased in the rats of the CMS-induced group, however, sucrose intake was restored in the rats of the CMS-induced depression and exercise group and in the CMS-induced depression and fluoxetine-treated group (Fig. 1).
Fig. 1.
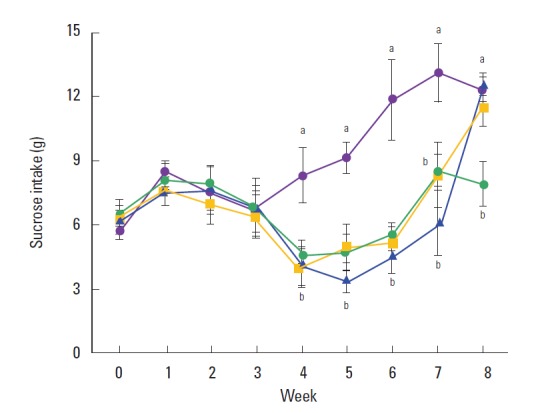
Effect of exercise on sucrose intake. (
 ) Control group, (
) Control group, (
 ) chronic mild stress (CMS)-induced depression group, (
) chronic mild stress (CMS)-induced depression group, (
 ) CMS-induced depression and exercise group, (
) CMS-induced depression and exercise group, (
 ) CMS-induced depression and fluoxetine-treated group. Letters (a, b) mean statistical significance P<0.05.
) CMS-induced depression and fluoxetine-treated group. Letters (a, b) mean statistical significance P<0.05.
Numbers of BrdU-positive cells
The number of BrdU-positive cells in the dentate gyrus was decreased in the rats of the CMS-induced depression group, however, the number of BrdU-positive cells was increased in the rats of the CMS-induced depression and exercise group and in the CMS-induced depression and fluoxetine-treated group (Fig. 2).
Fig. 2.
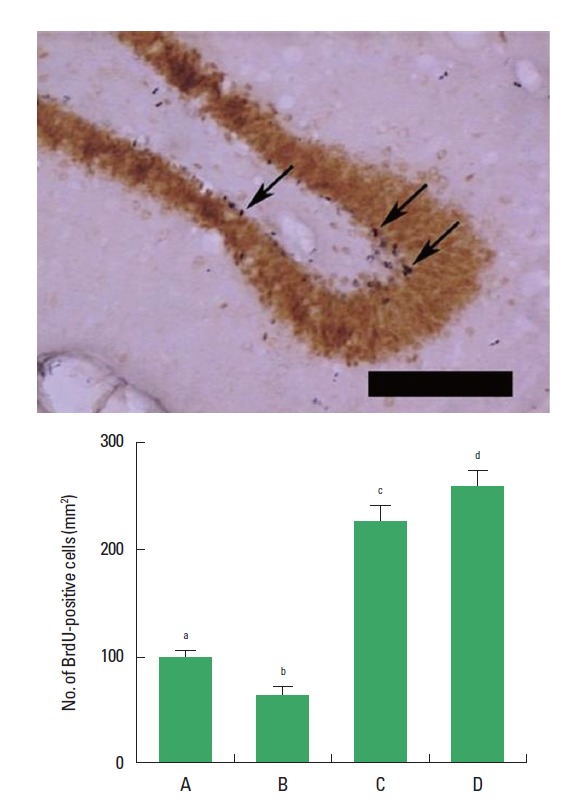
Effect of exercise on the number of 5-bromo-2′-deoxyuridine (BrdU)-positive cells in the dentate gyrusn. Upper: Photomicrographs of BrdU-positive cells in the dentate gyrus. Arrows indicate BrdU-positive cells. The scale bar represents 200 μm. Below: Number of BrdU-positive cells in each group. (A) Control group, (B) chronic mild stress (CMS)-induced depression group, (C) CMS-induced depression and exercise group, (D) CMS-induced depression and fluoxetine-treated group. Letters (a, b, c) mean statistical significance P<0.05.
Numbers of TUNEL-positive cells
The number of TUNEL-positive cells in the dentate gyrus was increased in the rats of the CMS-induced depression group, however, the number of TUNEL-positive cells was decreased in the rats of the CMS-induced depression and exercise group and in the CMS-induced depression and fluoxetine-treated group (Fig. 3).
Fig. 3.
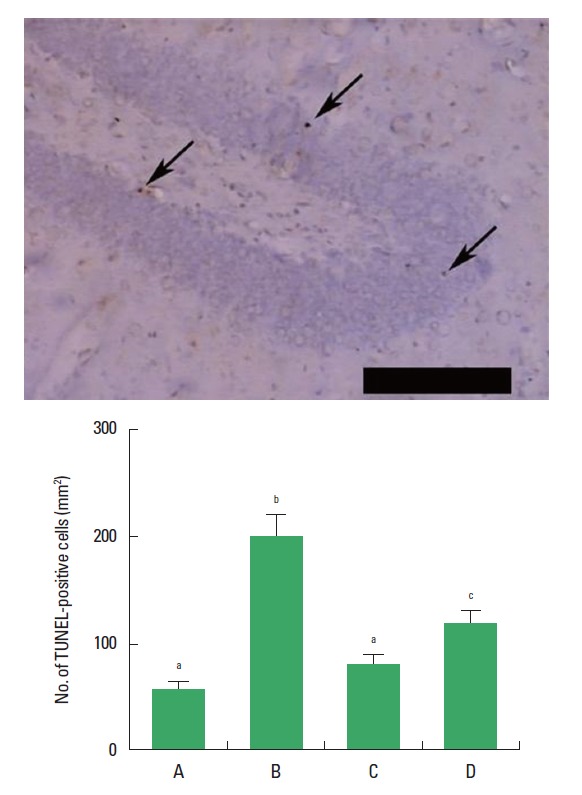
Effect of exercise on the number of terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL)-positive cells in the dentate gyrus. Upper: Photomicrographs of TUNEL-positive cells in the dentate gyrus. Arrows indicate TUNEL-positive cells. The scale bar represents 200 μm. Below: Number of TUNEL-positive cells in each group. (A) Control group, (B) chronic mild stress (CMS)-induced depression group, (C) CMS-induced depression and exercise group, (D) CMS-induced depression and fluoxetine-treated group. Letters (a, b, c) mean statistical significance P<0.05.
BDNF expression
The expression of BDNF in the dentate gyrus was decreased in the rats of the CMS-induced depression group, however, BDNF expression was increased in the rats of the CMS-induced depression and exercise group and in the CMS-induced depression and fluoxetine-treated group (Fig. 4).
Fig. 4.
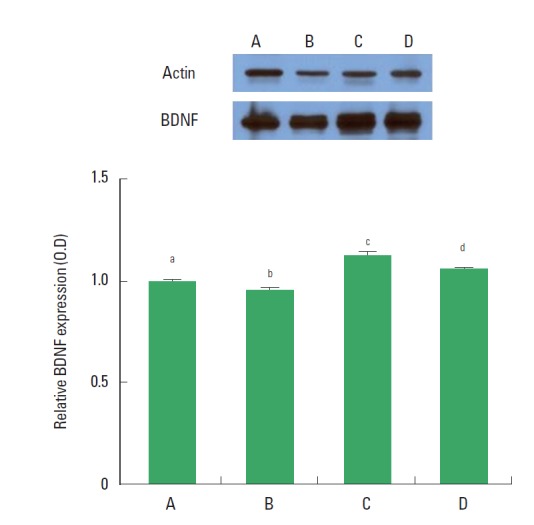
Western blot analysis of brain-derived neurotrophic factor (BDNF) expression in the dentate gyrus. (A) Control group, (B) chronic mild stress (CMS)-induced depression group, (C) CMS-induced depression and exercise group, (D) CMS-induced depression and fluoxetine-treated group. Letters (a, b, c, d) mean statistical significance P<0.05.
CREB expression
The expression of CREB in the dentate gyrus was not changed in the rats of the CMS-induced depression group compared to the control rats, however, CREP expression was increased in the rats of the CMS-induced depression and exercise group and in the CMS-induced depression and fluoxetine-treated group compared to the depression rats (Fig. 5).
Fig. 5.
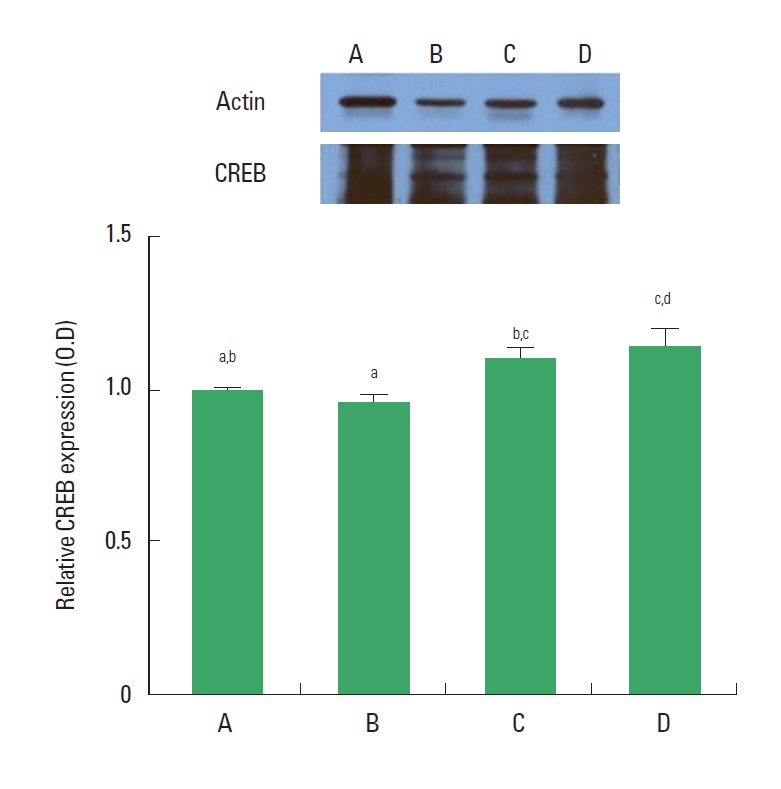
Actin Western blot analysis of expression level of cyclic adenosine monophosphate response element binding protein (CREB) expression in the dentate gyrus. (A) Control group, (B) chronic mild stress (CMS)-induced depression group, (C) CMS-induced depression and exercise group, (D) CMS-induced depression and fluoxetine-treated group. Letters (a, b, c, d) mean statistical significance P<0.05.
eNOS expression
The expression of eNOS in the dentate gyrus was not changed in the rats of the CMS-induced depression group compared to the control rats, however, eNOS expression was increased in the rats of the CMS-induced depression and exercise group compared to the depression rats (Fig. 6).
Fig. 6.
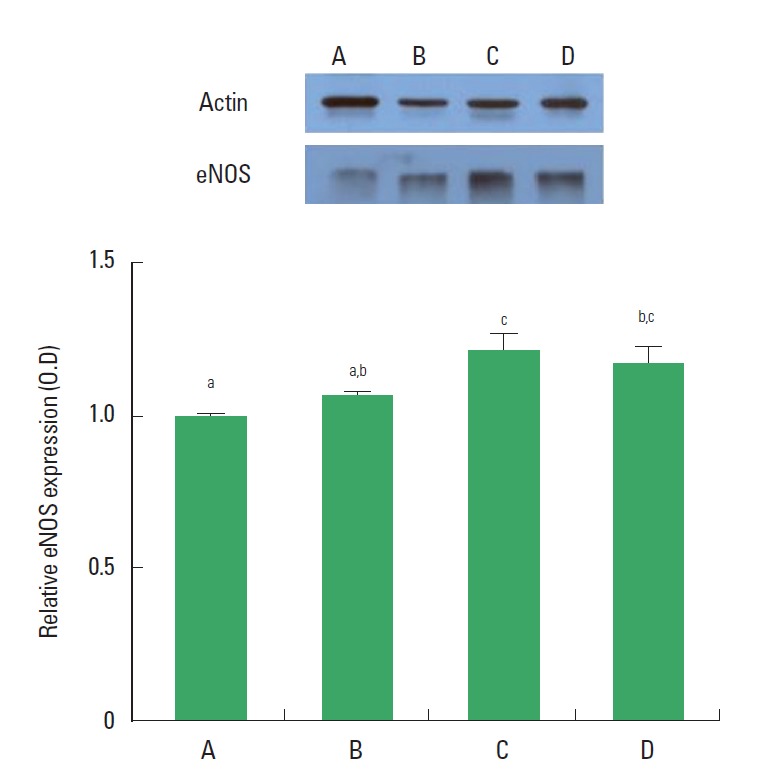
Actin Western blot analysis of expression level of endothelial nitric oxide synthase (eNOS) expression in the dentate gyrus. (A) Control group, (B) chronic mild stress (CMS)-induced depression group, (C) CMS-induced depression and exercise group, (D) CMS-induced depression and fluoxetine-treated group. Letters (a, b, c) mean statistical significance P<0.05.
DISCUSSION
In the present study, CMS procedure reduced sucrose intake of the rats, while exercise and fluoxetine-treatment increased CMS-induced decrement in sucrose intake. It was reported that anhedonia is one of the core symptoms of depression in humans (Rygula et al., 2005). This anhedonia demonstrate an operational change in reward sensitivity associated with CMS and they are in line with previous studies that have employed the CMS procedure (Grippo et al., 2005).
In the present study, CMS procedure reduced cell proliferation, while exercise and fluoxetine-treatment increased CMS-induced decrement in cell proliferation in the dentate gyrus. It was reported that stress and glucocorticoides impair hippocampal neurogenesis (Manji et al., 2003), and chronic stressed animals showed suppressed proliferation (Heine et al., 2004). Suppression of cell proliferation in the hippocampus could constitute one of the mechanisms of the depression (Bjørnebekk et al., 2005). Various experimental studies on the stress and anti-depressants indicate neurogenesis as the etiology of major depressive disorder (Kempermann and Kronenberg, 2003). Anti-depressant-like effect of running is associated with increased hippocampal cell proliferation (Bjørnebekk et al., 2005).
In the present study, CMS procedure increased the number of TUNEL-positive cells in the dentate gyrus, while exercise and fluoxetine-treatment decreased the number of TUNEL-positive cells. One of the most consistent effects of stress on cell morphology is atrophy of hippocampal neurons (Sapolsky, 2000). Several clinical studies indicated that a subset of patients with depression showed glucocorticoid hypersecretion or exhibited a hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis (Sapolsky, 2000). Moreover, reduction in hippocampal volume was seen in patients with HPA hyperactivity (Sapolsky, 2000). Stresses and elevated glucocorticoids induced glutamate excitotoxicity, disturbed calcium homeostasis, inhibited glucose transport, and increased oxygen radical generation (Sapolsky, 2000). Treadmill exercise is known to inhibit stress-induced apoptosis in the dentate gyrus (Kim and Seo, 2013).
In the present study, CMS procedure decreased expression of BDNF, while exercise and fluoxetine-treatment increased expression of BDNF and CREB in the dentate gyrus. Decreased BDNF level is a crucial phenomenon associated with stress, particularly relevant to stress-related depressive disorders (D’Sa and Duman, 2002). Dysfunction of the cAMP-CREB signaling cascade caused stress-induced BDNF down-regulation (Duman et al., 1997). The up-regulation of CREB expression was observed by administration of antidepressants and application of chronic electroconvulsive seizure (Duman et al., 2000). The brain cAMP signal transduction pathway is involved in the therapeutic action of antidepressants (D’Sa and Duman, 2002; Manji et al., 2003). Overexpression of CREB in the hippocampal dentate gyrus or infusion of BDNF into the hippocampus produced anti-depressant effect in animal models of depression (Shirayama et al., 2002). CREB is essential for long-term transcriptional changes associated with chronic antidepressant treatment (Conti et al., 2002). Therefore, antidepressants could mediate their effects by increasing neurogenesis and modulating the signaling pathways involved in plasticity and survival (D’Sa and Duman, 2002). Exercise alleviates stress-induced decrement in BDNF expression (Adlard and Cotman, 2004). Exercise-induced BDNF expression is associated with the expressions of several key intermediates of the phosphatidylinositol-3 kinase/Akt pathway, which is known to enhance neuronal survival (Chen and Russo-Neustadt, 2005).
In the present study, CMS procedure decreased expression of eNOS, while exercise increased expression of eNOS in the dentate gyrus. Brain NO has multiple functions, such as brain circuits and plasticity, neuroprotection and neurotoxicity, and behavior (Yermolaieva et al., 2000). Augmentation of NO production by eNOS increases cerebral blood flow, which exerts neuroprotection during brain ischemia (Hashiguchi et al., 2005). Neuroprotection by exercise is mediated by increased eNOS expression and augmentation of cerebral blood flow (Endres et al., 2003). eNOS in hippocampal blood vessels may diffuse into neuronal parenchyma to influence cell activity, and correlation between eNOS and neuronal activity was reported (Liu et al., 2005).
In the present study, treadmill exercise restored sucrose consumption, increased cell proliferation, and decreased apoptotic cell death. This antidepressive effect of treadmill exercise can be ascribed to the augmentation of cerebral blood flow through increasing eNOS expression, and then this may increase BDNF expression.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010-0022895).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A, Mathé AA, Brené S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol. 2005;8:357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Mellédo JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry. 2004;56:129–134. doi: 10.1016/j.biopsych.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado PL. Approaches to the enhancement of patient adherence to antidepressant medication treatment. J Clin Psychiatry. 2000;61( Suppl 2):6–9. [PubMed] [Google Scholar]
- D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17( Suppl 3):306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schröck H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czéh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14( Suppl 5):S481–490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 2005;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Hashiguchi A, Yano S, Morioka M, Hamada J, Kochi M, Fukunaga K. Dephosphorylation of eNOS on Thr495 after transient forebrain ischemia in gerbil hippocampus. Brain Res Mol Brain Res. 2005;133:317–319. doi: 10.1016/j.molbrainres.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joëls M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Heo YM, Shin MS, Kim SH, Kim TW, Baek SB, Baek SS. Treadmill exercise ameliorates disturbance of spatial learning ability in scopolamine-induced amnesia rats. J Exerc Rehabil. 2014;10:155–161. doi: 10.12965/jer.140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Jung SB, Lee TH, Bahn GH, Kwon YK, Kim EH, Kim CJ. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport. 2002;13:1509–1513. doi: 10.1097/00001756-200208270-00004. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet. 2000;355:911–918. doi: 10.1016/S0140-6736(99)11381-3. [DOI] [PubMed] [Google Scholar]
- Kim BK, Seo JH. Treadmill exercise alleviates post-traumatic stress disorder-induced impairment of spatial learning memory in rats. J Exerc Rehabil. 2013;9:413–419. doi: 10.12965/jer.130058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Lim BV, Kim K, Seo JH, Kim CJ. Treadmill exercise alleviates stress-induced impairment of social interaction through 5-hydroxytryptamine 1A receptor activation in rats. J Exerc Rehabil. 2015;11:192–197. doi: 10.12965/jer.150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Jang MH, Shin MC, Lim BV, Kim YP, Kim H, Choi HH, Lee KS, Kim EH, Kim CJ. Dependence of rat hippocampal c-Fos expression on intensity and duration of exercise. Life Sci. 2003;72:1421–1436. doi: 10.1016/s0024-3205(02)02406-2. [DOI] [PubMed] [Google Scholar]
- Liu P, Smith PF, Appleton I, Darlington CL, Bilkey DK. Hippocampal nitric oxide synthase and arginase and age-associated behavioral deficits. Hippocampus. 2005;15:642–655. doi: 10.1002/hipo.20085. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, A Gray N, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim YJ. Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer’s disease rats. J Exerc Rehabil. 2014;10:81–88. doi: 10.12965/jer.140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. J Clin Psychiatry. 1998;59( Suppl 18):23–29. [PubMed] [Google Scholar]
- Yermolaieva O, Brot N, Weissbach H, Heinemann SH, Hoshi T. Reactive oxygen species and nitric oxide mediate plasticity of neuronal calcium signaling. Proc Natl Acad Sci U S A. 2000;97:448–453. doi: 10.1073/pnas.97.1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]


