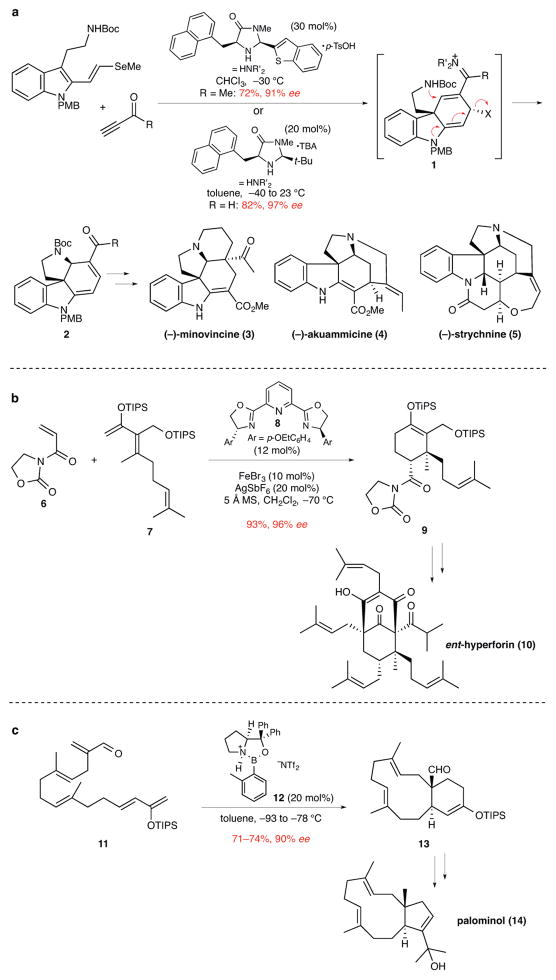
Figure 2. The use of catalytic enantioselective Diels–Alder reactions to synthesize natural products containing quaternary stereocenters.
ee, enantiomeric excess. a, A bimolecular Diels–Alder reaction promoted by iminium ion activation forms intermediate 1 in the first step of a cascade sequence generating tetracyclic product 2. This product contains the quaternary stereocenter and four rings common to several groups of indole alkaloids and was employed to complete enantioselective total syntheses of various indole alkaloids, including (−)-minovincine (3), (−)-akuammicine (4), and (−)-strychnine (5).7 Boc, tert-butoxycarbonyl; Me, methyl; PMB, p-methoxybenzyl; p-TsOH, p-toluenesulfonic acid; t-Bu, tert-butyl; TBA, tribromoacetic acid. b, An iron-bisoxazoline catalyzed bimolecular Diels–Alder reaction forms product 9 whose quaternary stereocenter subsequently controlled the elaboration of the two additional quaternary stereocenters of ent-hyperforin (10).11 TIPS, triisopropylsilyl; Et, ethyl; MS, molecular sieves. c, Oxazaborolidinium-catalyzed intramolecular Diels–Alder reaction to form the 11-membered ring and quaternary stereocenter of palominol (14).12 Tf, trifluorosulfonyl; TIPS, triisopropylsilyl; Ph, phenyl.