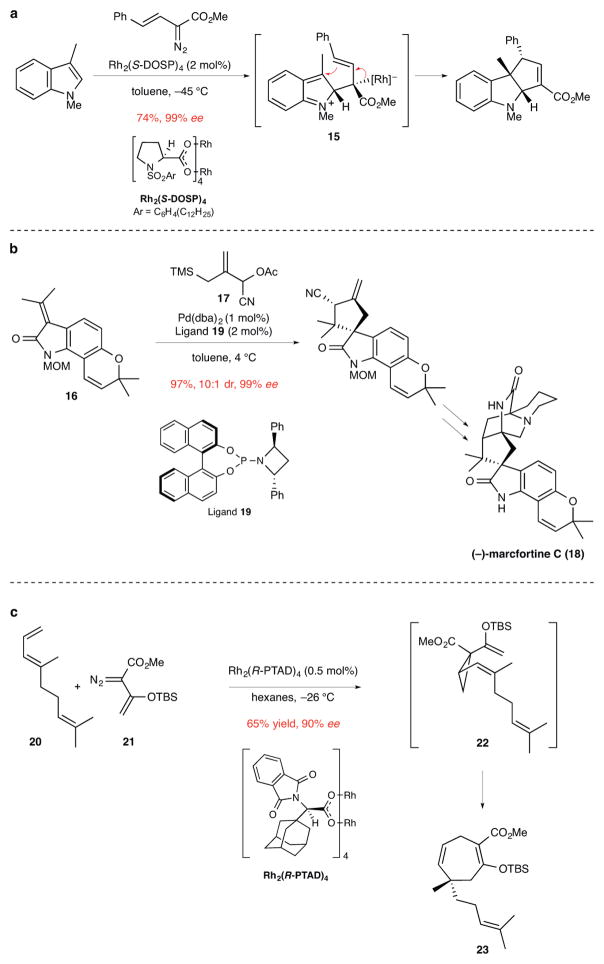
Figure 3. Examples of other catalytic enantioselective cycloaddition reactions used to prepare products containing quaternary stereocenters.
ee, enantiomeric excess. a, The synthesis of a cyclopentene-fused indoline by a formal [3+2]-cycloaddition of 1,3-dimethylindole and a vinyl diazoester using a rhodium catalyst. This reaction is suggested to take place in a stepwise fashion via dipolar intermediate 15.14 Me, methyl; Ph, phenyl. b, The [3+2]-cycloaddition of a Pd-trimethylenemethane intermediate generated from allylic acetate 17 to form a tetracyclic intermediate in the total synthesis of (−)-marcfortine C.16 MOM, methoxymethyl; TMS, trimethylsilyl; Ac, acetate; dba, dibenzylideneacetone; Ph, phenyl. c, Enantioselective synthesis of 1,4-cycloheptadiene 23 from triene 20 and vinyl diazoester 21. The first step in this sequence is Rh-catalyzed cyclopropanation of the terminal double bond of the acyclic triene to form divinyl cyclopropane 22, which upon in situ Cope rearrangement generates 23 and its quaternary stereocenter. Product 23 was employed in the total synthesis of the diterpenoid (−)-5-epi-vibsanin E.21 Me, methyl; TBS, tert-butyldimethylsilyl.