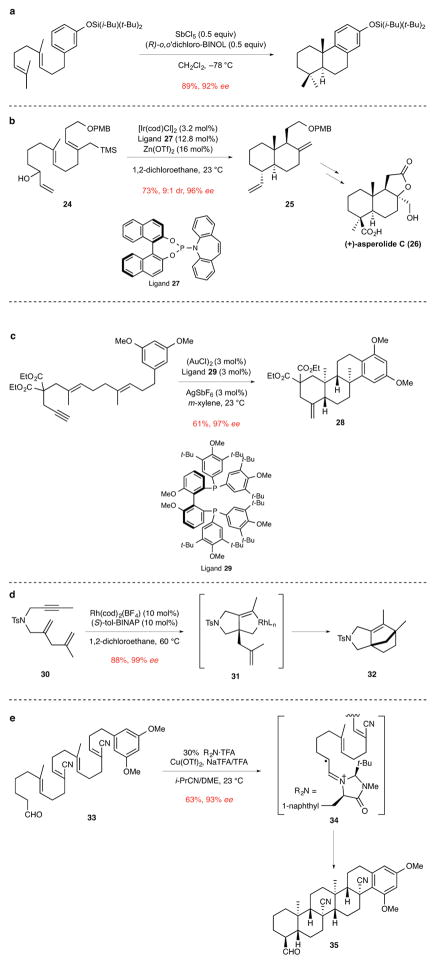
Figure 4. Catalytic enantioselective polyene cyclizations to construct polycyclic products having quaternary stereocenters.
ee, enantiomeric excess. a, The use of a protic acid catalyst for the cyclization of an aryl diene to form two rings and one quaternary stereocenter.24 i-Bu, isobutyl; t-Bu, tert-butyl; BINOL, 1,1′-bi-2-naphthol. b, The iridium-catalyzed cyclization of a triene alcohol to construct the trans-decalin core 25 of the labdane diterpenoid (+)-asperolide C (26). The first step in this cascade cyclization is the generation of a η3-allyliridium cation from the allylic alcohol fragment of 24.26 PMB, p-methoxybenzyl; TMS, trimethylsilyl; cod, 1,5-cyclooctadiene; Tf, trifluorosulfonyl. c, The gold-catalyzed cyclization of an aryl dienyne to form three rings and two quaternary stereocenters of tetracyclic product 28.27 Et, ethyl; Me, methyl; t-Bu, tert-butyl. d The rhodium-catalyzed cyclization of dienyne 30 to form bridged azatricyclic product 32. This reaction is suggested to take place via metallacyclic intermediate 31, which undergoes alkene insertion and reductive elimination to furnish product 32.30 Ts, p-toluenesulfonyl; tol-BINAP, 2,2′-bis(di-p-tolylphosphino)-1,1′-binaphthalene; cod, 1,5-cyclooctadiene; L, ligand. e, The cyclization of tetraene aldehyde 33 in the presence of an imidazolone catalyst and a Cu(II) oxidant to form five rings and four quaternary stereocenters of hexacyclic product 35. This novel reaction is suggested to proceed by single-electron oxidation of the initially formed iminium ion intermediate to generate 34, which undergoes a series of 6-endo radical cyclizations to eventually give product 35. The nitrile substituents are incorporated to disfavor 5-endo cyclizations in the formation of the second and fourth rings.32 Me, methyl; Tf, trifluorosulfonyl; TFA, trifluoroacetic acid; NaTFA, sodium trifluoroacetate; i-Pr, isopropyl; DME, 1,2-dimethoxyethane.