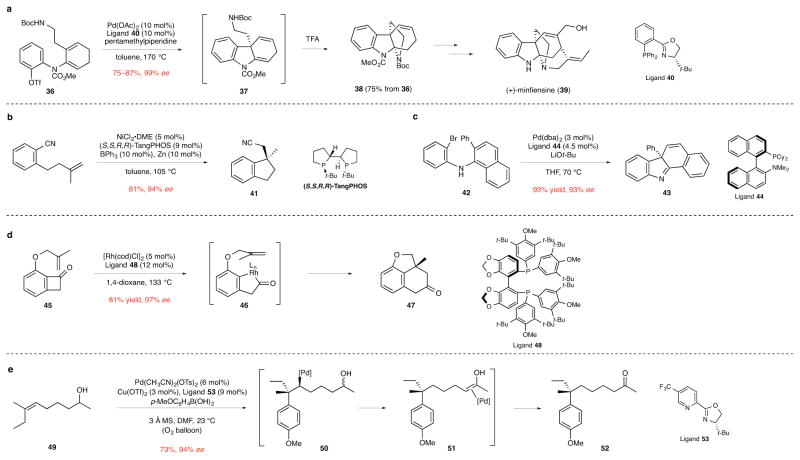
Figure 5. Transition metal-catalyzed insertion reactions that form quaternary stereocenters.
ee, enantiomeric excess. a, The enantioselective intramolecular Heck cyclization of dienyl triflate 36 to form 1,4-diene intermediate 37, which upon exposure to excess trifluoroacetic acid provided tetracyclic product 38 in route to the indole alkaloid (+)-minfiensine (39). The use of PHOX ligand 40 was critical in achieving both high stereoinduction and preventing isomerization of the initially formed product 37 to the conjugated 1,3-diene regioisomer.33 Boc, tert-butoxycarbonyl; Me, methyl; Tf, trifluorosulfonyl; Ac, acetyl; TFA, trifluoroacetic acid. b, The intramolecular nickel-catalyzed arylcyanation of a tethered double bond to form indane 41.34 DME, 1,2-dimethoxyethane; Ph, phenyl; t-Bu, tert-butyl. c, The palladium-catalyzed cyclization/dearomatization of aryl(naphthyl)amine 42 to form tetracyclic product 43. This reaction is suggested to occur via a six-membered palladacyclic intermediate that undergoes reductive elimination to form generate product 43.36 Ph, phenyl; dba, dibenzylideneacetone; t-Bu, tert-butyl; THF, tetrahydrofuran; Me, methyl; Cy, cyclohexyl. d, The rhodium-catalyzed conversion of alkenyl benzocyclobutanone 45 to tricyclic ether 47. This transformation is believed to occur by initial insertion of rhodium into the C–C bond to form acylrhodium intermediate 46, which in the enantiodetermining step undergoes intramolecular carboacylation of the tethered alkene to form product 47.37 cod, 1,5-cyclooctadiene; L, ligand; Me, methyl; t-Bu, tert-butyl. e, The bimolecular Heck-type addition of an arylboronic acid to the trisubstituted double bond of 49 to form ketone product 52. This rare example of a bimolecular alkene insertion to form a quaternary stereocenter is suggested to occur by initial enantioselective carbopalladation of the alkene to generate intermediate 50, which undergoes sequential β-hydride eliminations/migratory insertions along the alkyl chain to form alkene complex 51 and then the ketone product.38 Ts, p-toluenesulfonyl; Tf, trifluorosulfonyl; Me, methyl; MS, molecular sieves; DMF, N,N-dimethylformamide; t-Bu, tert-butyl.