Figure 8. Use of palladium-catalyzed asymmetric allylic alkylation reactions for constructing quaternary centers in alkaloid and terpenoid natural products.
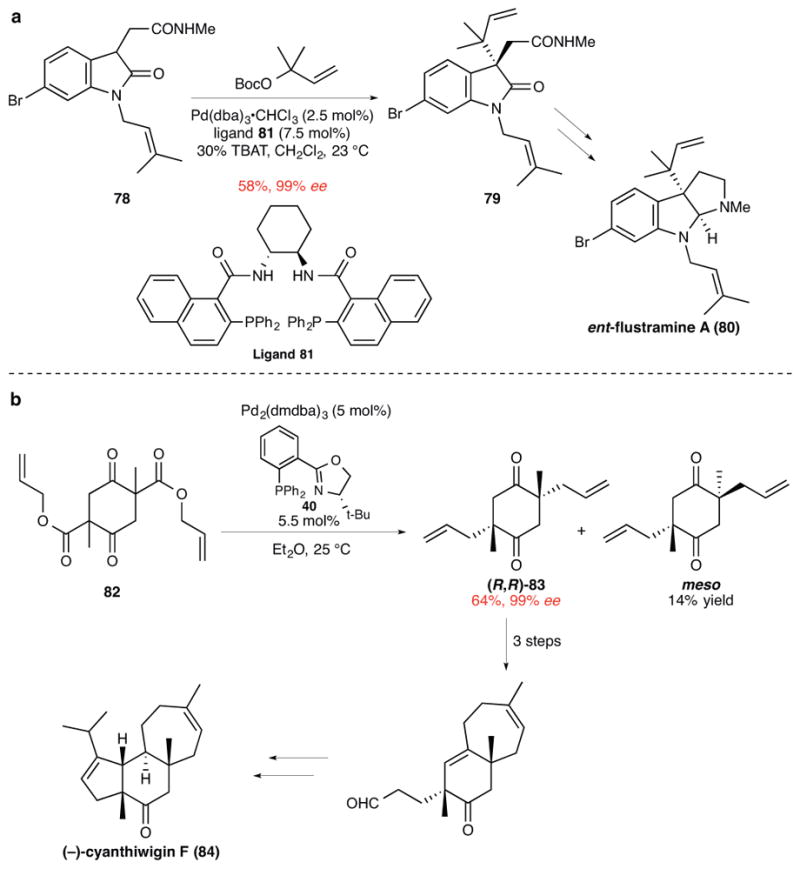
a, The regioselective prenylation of oxindole 78 upon base-promoted reaction with the η3-allylpalladium electrophile generated from a prenyl carbonate to form 79. This product was a late-stage intermediate in the enantioselective total synthesis of ent-flustramine A (80).69 Me, methyl; Boc, tert-butoxycarbonyl; dba, dibenzylideneacetone; TBAT, tetrabutylammonium difluorotriphenylsilicate; Ph, phenyl. b, The syn-diastereoselective diallylation of β-ketoester 82 (a mixture of racemic diastereomers) to give (R,R)-83, a pivotal intermediate in the enantioselective total synthesis of (−)-cyanthiwigin F.70 dmdba, bis(3,5-dimethoxybenzylidene)acetone; Ph, phenyl; t-Bu, tert-butyl.
