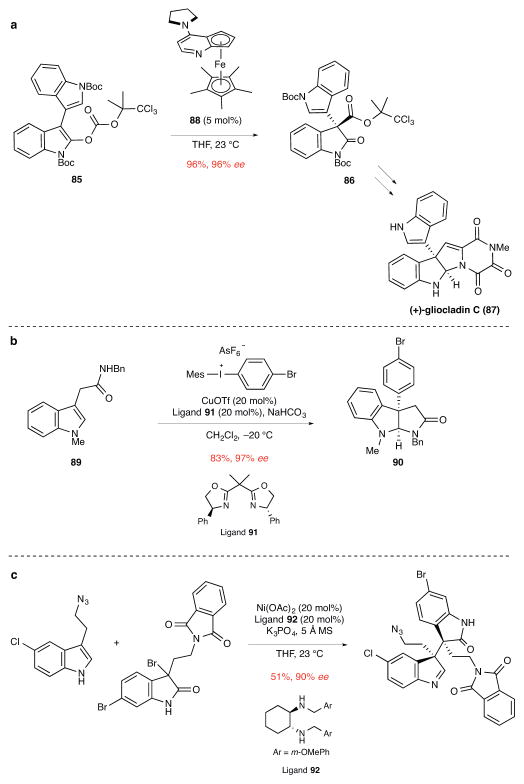
Figure 9. Miscellaneous methods involving the union of a catalytically generated chiral carbon electrophile with a carbon nucleophile.
ee, enantiomeric excess. a, The Steglich rearrangement of indole carbonate 85 in the presence of Fu’s planar-chiral catalyst 88 to give 3,3-disubstituted oxindole 86 in route to (+)-gliocladin C.78 Boc, tert-butoxycarbonyl; Me, methyl; THF, tetrahydrofuran. b, The copper-catalyzed β-arylation of indole 89 and concomitant cyclization to form 3a-arylpyrrolidinoindolinone 90.80 Me, methyl; Bn, benzyl; Tf, trifluorosulfonyl; Mes, 1,3,5-trimethylbenzene; Ph, phenyl. c, The Ni-catalyzed coupling of an indole with a 3-bromooxindole in route to (+)-perophoramidine. This reaction sets the two contiguous quaternary stereocenters of (+)-perophoramidine.85 OAc, acetoxy; MS, molecular sieves; THF, tetrahydrofuran; Me, methyl; Ph, phenyl.