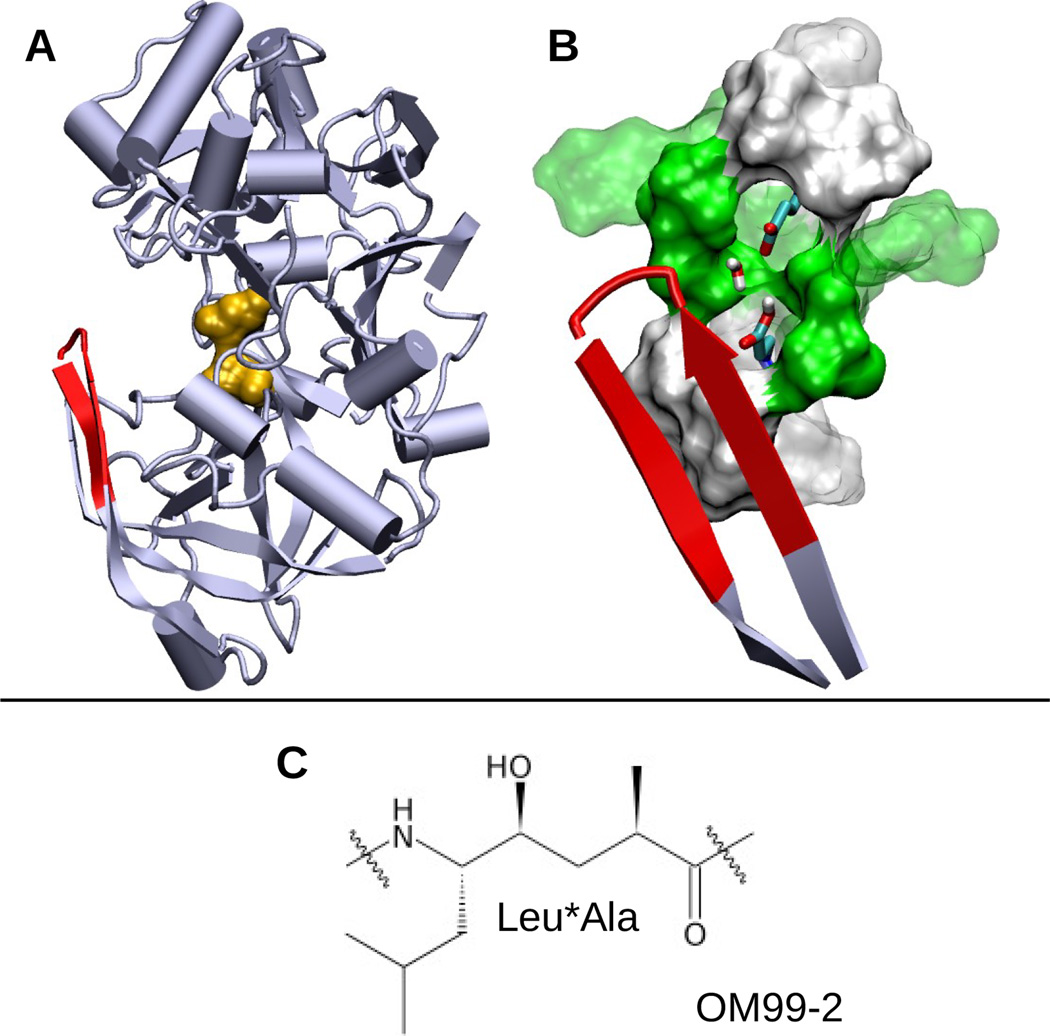
Figure 1.
Structures of BACE1 and the inhibitor OM99-2. A. Cartoon representation of apo BACE1 (PDB ID: 1SGZ) with the catalytic dyad (Asp32 and Asp228) colored orange and the flap (β-hairpin loop, residues 68–77) colored red. B. Active site of BACE1. Asp32, Asp228 and bridging water are shown in stick model. Polar (green) and hydrophobic (white) residues within 5 Å and 7 Å of Asp32/Asp228 are depicted as solid and transparent surfaces, respectively. C. The central hydroxyethylene group (Leu*Ala) of the peptidomimetic inhibitor OM99-2. The entire sequence is Glu-Val-Asn-Leu*Ala-Ala-Glu-Phe.