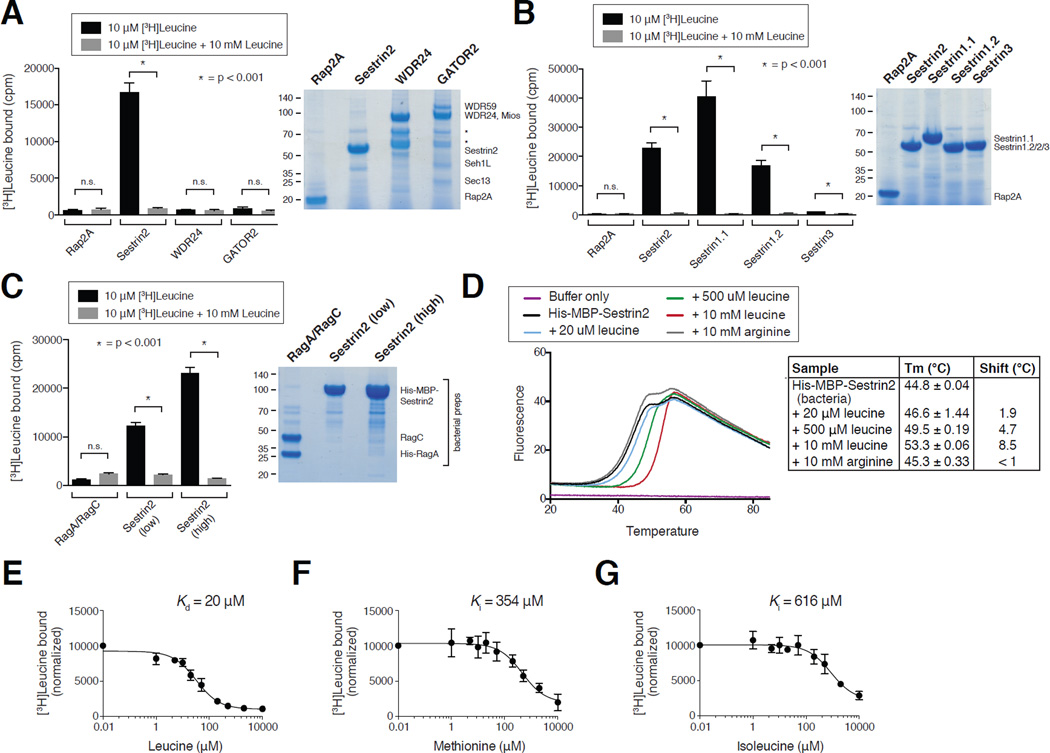
Figure 2. Sestrin2 binds leucine with a Kd of 20 µM.
A) Binding of radiolabeled leucine to Sestrin2, but not WDR24, GATOR2, or the control protein Rap2A. FLAG immunoprecipitates prepared from HEK-293T cells transiently expressing indicated proteins or complexes were used in binding assays with [3H]Leucine as described in the methods. Unlabeled leucine was added where indicated. Values are Mean ± SD for 3 technical replicates from one representative experiment. SDS-PAGE followed by Coomasie blue staining was used to analyze immunoprecipitates prepared in parallel to those included in the binding assays. Asterisks indicate breakdown products in the WDR24 and GATOR2 purifications.
B) Leucine-binding capacities of Sestrin1 (two isoforms), Sestrin2, and Sestrin3. FLAG immunoprecipitates were prepared and binding assays performed and analyzed as in (A).
C) Leucine binds to bacterially-produced Sestrin2, but not the RagA/RagC heterodimer. Leucine binding assays were performed as described in the methods and analyzed as in (A) with His- MBP-Sestrin2 or His-RagA/RagC bound to the Ni-NTA resin.
D) Effects of leucine and arginine on the melting temperature of bacterially-produced Sestrin2 in a thermal shift assay. His-MBP-Sestrin2 was incubated with the Sypro orange dye with or without leucine or arginine. Upon heating the sample the change in fluorescence was captured and used to calculate melting temperatures (Tm) under the indicated conditions. Values are Mean ± SD from 3 replicates.
E) Sestrin2 binds leucine with a Kd of 20 µM. FLAG-Sestrin2 immunoprecipitates prepared as in (A) were used in binding assays with 10 µM or 20 µM [3H]Leucine and indicated concentrations of unlabeled leucine. In the representative graph shown each point represents the normalized mean ± SD for n = 3 in an assay with 10 µM [3H]Leucine. The Kd was calculated from the results of six experiments (three with 10 µM and three with 20 µM [3H]Leucine).
F) Methionine can compete the binding of leucine to Sestrin2. FLAG-Sestrin2 immunoprecipitates prepared as in (A) were used in binding assays with 10 µM [3H]Leucine and indicated concentrations of unlabeled methionine. In the graph shown each point represents the normalized mean ± SD for n = 3. The Ki was calculated using data from the three experiments.
G) Isoleucine can compete the binding of leucine to Sestrin2. FLAG-Sestrin2 immunoprecipitates prepared as in (A) were used in binding assays with 10 µM [3H]Leucine and indicated concentrations of unlabeled isoleucine. In the graph shown each point represents the normalized mean ± SD for n = 3. The Ki was calculated using data from the three experiments.