Abstract
Previous studies in rat hepatocytes demonstrated that insulin-dependent apolipoprotein (apo) B degradation (IDAD) is lost when cells are maintained for 3 d under enriched culture conditions. Loss of IDAD correlates with increased expression of protein tyrosine phosphatase 1B (PTP1B) known to be associated with resistance to insulin signaling in the liver. McArdle RH7777 hepatoma (McA) cells cultured in serum containing medium are resistant to IDAD; demonstrate a 30% increase in apo B secretion, and express increased levels of PTP1B protein and mRNA. In addition, insulin-stimulated Class I phosphatidylinositide 3-kinase (PI3K) activity of anti-pY immunoprecipitates is severely blunted. IDAD resistance in McA cells correlates with diminished translocation of insulin-stimulated pY-IRS1 to intracellular membranes. Incubation of McA cells with RK682, a protein tyrosine phosphatase inhibitor, is sufficient to restore IDAD in resistant McA cells. Overall, results further support the importance of Class I PI3K activity in IDAD, and suggest that loss of this activity is sufficient to cause resistance. Although other factors are involved in downstream events including sortilin binding to apo B, autophagy, and lysosomal degradation, loss of signal generation and reduced localization of Class I PI3K to intracellular membranes plays a significant role in IDAD resistance.
Keywords: apo B, PI3K, IRS1, IRS2, PTP1B, VLDL, liver
1. Introduction
Understanding mechanisms involved in VLDL hypersecretion is important as it underlies the development of hypertriglyceridemia associated with metabolic syndrome [1]. The focus of our laboratory has been to determine mechanism(s) of insulin-dependent apo B degradation (IDAD) using rat hepatocytes (RH) and liver-derived McArdle RH7777 (McA) cells as model systems in order to understand factors responsible for hypersecretion of VLDL. We first reported IDAD in 1990 showing in RH that in response to insulin, VLDL apo B secretion was suppressed by presecretory degradation of B100 preferentially over B48 [2]. In RH insulin stimulates tyrosine phosphorylation (pY) of insulin receptor substrate 1 (IRS1) and translocation of activated Class I PI3K to the endoplasmic reticulum (ER), the site of VLDL assembly [3]. The suppressive effect of insulin has since been demonstrated in humans [4,5,6]. IDAD plays a role in minimizing hepatic VLDL secretion during the post-prandial period to limit competition with triglyceride-rich lipoproteins for catabolism [7]
IDAD does not involve reduced MTP activity [8], or LDL receptor-mediated degradation [9]. In McA cells constitutively active Class I PI3K suppresses apo B secretion supporting the importance of Class I PI3K [10]. Insulin-mediated translocation of PI3K and generation of phosphatidylinositide (3,4,5) triphosphate (PIP3) occurs rapidly, and likely precedes insulin-dependent B100 binding to sortilin [10], a Golgi-localized, multi-ligand sorting receptor [11]. The specific requirement for PIP3 generated by Class I PI3K is supported by loss of IDAD with PTEN overexpression [12]. Complete degradation of B100 also involves the Class III PI3K, Vps34, and autophagy followed by lysosomal degradation [12,13]. Class II PI3K has also been implicated as an additional factor in IDAD [13]
Resistance to IDAD and VLDL hypersecretion occurs in obese Zucker rats [14], rats and humans with insulin resistance and type 2 diabetes [5,6,15], fructose-fed B100 mice and hamsters [16,17]. The current investigation was undertaken to explore factors in IDAD resistance potentially responsible for hepatic VLDL hypersecretion.
2. Materials and methods
2.1. Cell lines, materials and reagents
McArdle RH-7777 cells were obtained from the American Type Culture Collection (ATCC® CRL-1601™, Manassas, VA). Materials and reagents were essentially as described previously [10]. RK-682 was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY). Phosphatase inhibitor cocktail II and protease inhibitor cocktail III were obtained from Calbiochem (San Diego, CA). PROTEAN®TGX™ (TGX) SDS polyacrylamide gels (4–15% acrylamide) and PVDF membranes were obtained from Bio-Rad Laboratories (Hercules, CA). Mouse anti-pY monoclonal antibody (4G10) and rabbit anti-IRS1, IRS2, PTP1B and p85 were from Millipore (Temecula, CA). Mouse anti-GAPDH antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-mouse and anti-rabbit horseradish peroxidase (HRP)-linked IgG, ECL reagents, and Amersham™ Hyperfilm™ were from GE Healthcare (Buckinghamshire, UK). For PI3K assays, substrate phosphatidylinositol (PI) was obtained from Avanti Polar Lipids (Alabaster, AL), and [γ-32P]ATP (3000 Ci/mmol) was from Perkin Elmer-NEN Life Science Products (Waltham, MA).
2.2. Animals and primary rat hepatocyte cultures
All rat experimental procedures were approved by the University of Rochester School of Medicine & Dentistry’s Animal Use and Care Committee (Rochester, NY). Primary RH were prepared from Sprague Dawley rats using methods as detailed [18,19]. Purified RH were seeded on collagen coated dishes for 4 h, and then incubated for 1 d (24 h) and up to 3 d (72 h) in enriched Waymouth’s MB 752/1 medium containing 0.2% (w/v) BSA, added amino acids, dexamethasone (1 μM), lactate (10 mM), pyruvate (1 mM), and sodium oleate (0.75 mM) [20] with replacement of fresh medium every 24 h. RH terminated 4 h after seeding were considered the 0 d time point, whereas RH terminated at the end of each 24-h period corresponded to incubations of 1 d, 2 d, and 3 d. Incubations were terminated by rinsing cells three times in HBSS followed by freezing cells in liquid nitrogen and storage at −80 °C until further analysis.
2.3. McArdle RH-7777 cell culture
McA cells were cultured under enriched culture conditions in complete DMEM (cDMEM) containing 10% (v/v) fetal bovine serum (FBS), 10% (v/v) horse serum, and antibiotics [21]. For serum-free conditions, McA cells having reached 60–70% confluence were rinsed three times in HBSS containing 0.2% (w/v) BSA, and re-incubated overnight (14–18 h) in DMEM containing 1% (w/v) BSA (1% BSA/DMEM). McA cell incubations were terminated as described for RH.
2.4. Western immunoblotting
RH or McA cells were scrape/thawed on ice in ice-cold lysis buffer containing 1% (v/v) NP40, 25 mM Tris-HCl (pH 7.4), 10% (v/v) glycerol, 150 mM NaCl, 2 mM EDTA, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1% (v/v) aprotinin, 1% (v/v) phosphatase inhibitor cocktail II, 1% (v/v) protease inhibitor cocktail III, and 1 mM AEBSF. Cellular debris was removed by centrifugation at 17,000 x g for 15 min at 4 °C. Lysate protein content was determined by bicinchoninic acid (BCA) assay (Pierce Corp.). Proteins were denatured by addition of 4X Laemmli’s gel loading buffer containing dithiothreitol (final concentration, 10 mM), and heating to 95 °C for 10 min. Cellular protein levels were determined by western immunoblotting after loading equivalent protein per lane, protein separation by SDS-PAGE and electrophoretic transfer to PVDF membranes [10]. After incubation with primary antibodies, membranes were incubated with secondary HRP-linked antibodies followed by chemiluminescence detection (ECL) and Amersham™ Hyperfilm™ or imaged using ChemiDocXRS+ system (Bio-Rad). Exposures were digitized and band intensities quantified using Image Lab 3.0.1 software (Bio-Rad).
2.5. RNA isolation and mRNA quantitation
Total RNA was extracted from McA cells using TriZol Reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. Genomic DNA was removed using the TURBO DNA-free kit (Life Technologies), and RNA quality was assessed using the Agilent 2100 BioAnalyzer (Agilent Technologies, Palo Alto, CA). cDNA synthesis was performed using the High-Capacity cDNA reverse Transcription Kit (Life Technologies). PCR reactions were performed in triplicate (40 cycles) on the ABI 7900HT real time PCR instrument by University of Rochester Genomic Research Center and analyzed using the ΔΔCt relative quantitation method normalizing results to 36B4 expression. TaqMan® gene expression primer probe sets used for fluorogenic quantification of rat mRNA transcripts were: Irs1, Rn02132493; Irs2, Rn01482270: Pik3r1 (p85α), Rn00564547; Pik3r2 (p85β) Rn00573376; Ptpn1 (PTP1B), Rn01423685, and Rplp0 (ARBP, 36B4), Rn00821065.
2.6. Subcellular fractionation
Subcellular fractionations were carried out in a paired fashion where McA cells incubated in cDMEM were fractionated side-by-side with corresponding McA cells in 1% BSA/DMEM. McA cells (10-cm2 dishes or 150-cm2 flasks) were scraped into 0.25 M sucrose, 20 mM Tris-HCl (pH 7.5), 1 mM EDTA homogenizing buffer with added protease and phosphatase inhibitors [3], and cells were disrupted using a ball bearing homogenizer (Isobiotech, Heidelberg, Germany) using 10 strokes (20-micron clearance). Plasma membranes (PM), mitochondria and nuclei (PMN) were removed by centrifugation at 17,000 x g for 15 min at 4 °C. The high-speed pellet (HSP) and supernatant cytosol were then separated by centrifugation at 200,000 x g for 75 min at 4 °C. PM fractions were isolated at the interface following layering the resuspended PMN fraction on a 38% (w/v) sucrose cushion and centrifugation at 100,000 x g for 60 min at 4 °C. Fractions were adjusted to contain 1% (v/v) NP-40), and pY-phosphorylated proteins were immunoprecipitated overnight at 4 °C using anti-pY antibody (4G10). Immune complexes were collected using protein G-sepharose and incubation for 3–4 h at 4 °C [3]. After extensive washing, immunoprecipitates were eluted in 2X Laemmli’s buffer by heating at 95°C for 10 min and eluted proteins were stored at −80°C until analysis.
2.7. In vitro PI3K activity assay
In a paired fashion, McA cells in cDMEM or 1% BSA/DMEM were treated with insulin (0–1000 nM) for 5 min; media were removed and cells were washed three times in ice-cold HBSS containing 100 μM vanadate. Cells were solubilized in 1% (v/v) NP40 plus phosphatase and protease inhibitors (as above), and cellular debris and nuclei were removed by centrifugation at 17,000 x g for 15 min at 4 °C. Clarified lysates were incubated with anti-pY antibody and washed immunoprecipitates were assayed for PI3K activity using phosphatidylinositol substrate and radioactive AT32P as described previously [3]. Radioactive PI3P was quantified by PhosphoImager analysis (Molecular Dynamics, Inc., Sunnyvale, CA), and normalized to lysate protein.
2.8. Apo B Radioimmunoassay (RIA)
Media apo B concentrations were determined by competitive RIA using rat VLDL apo B as standard and 125I-labeled mouse monoclonal antibody equally reactive to rat B100 and B48 [22]. Apo B concentrations were normalized to cell protein as quantified by BCA protein assay (Pierce, Rockland, IL).
2.9. Statistics
Unless noted, results are expressed as the mean ± S.E.M., where n equals the number of independent experiments in which replicate analyses were performed in each experiment. Significant differences were assessed using Student’s t-test with p-values ≤ 0.05 being considered significant.
3. Results and discussion
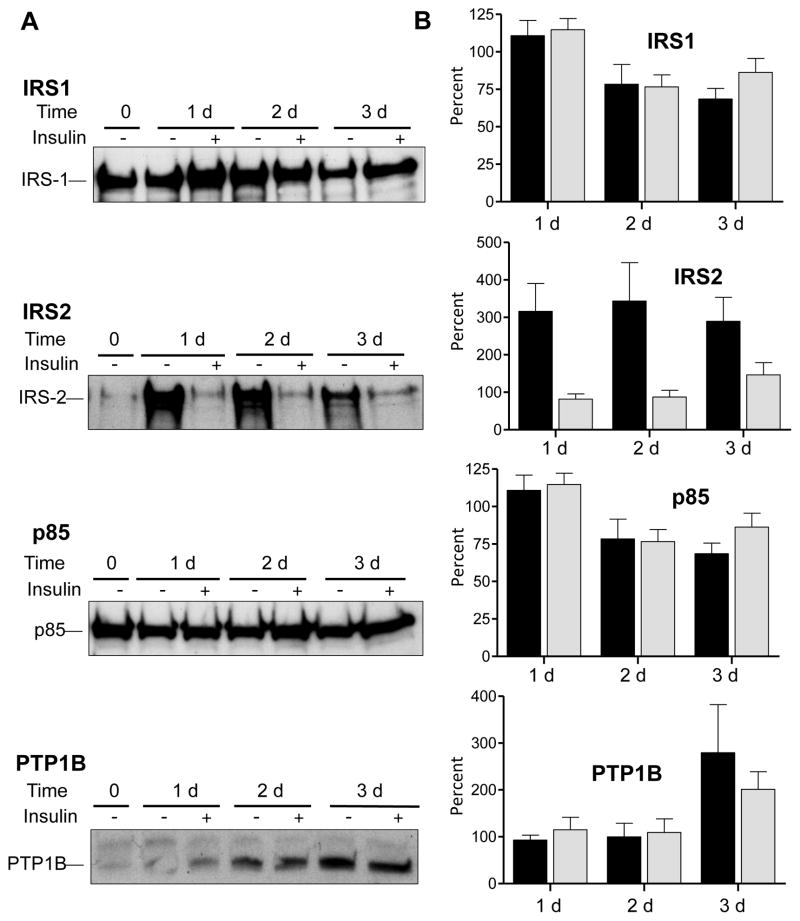
To study IDAD resistance we first compared insulin signaling molecules in primary RH sensitive to insulin (1 d) to those in RH that develop resistance by 3 d of incubation in enriched medium [20]. As seen in Fig. 1, only modest reductions in IRS1 and p85, the regulatory subunit of Class I PI3K, protein were observed over 3 d in the presence or absence of insulin. IRS2 was significantly elevated when RH were incubated in the absence of insulin as observed by others [23]. By the third d, IRS2 protein was increased 2.9 ± 0.6 times that present at d 1 (p < 0.05, n = 4). Elevated IRS2 levels were diminished when RH were incubated with insulin. By d 3 when RH develop IDAD resistance, PTP1B protein was increased whether or not insulin was included in the medium. By d 3, PTP1B was increased 2.8 ± 1.0 times that present at d 1 in the absence of insulin (p < 0.05, n = 4), and 2.0 ± 0.4 times that present at d 1 (p < 0.05, n = 4) in the presence of insulin. Results without insulin indicate that RH resistant to IDAD at 3 d express significantly increased levels of IRS2 and PTP1B (Fig. 1B). IRS2 levels were less at 1d than at 3 d (reduced by 67% ± 13.5%, n = 6, p < 0.005). PTP1B levels were less at 1 d than 3 d (reduced by 58% ± 6.5%, n = 4, p < 0.005).
Fig. 1. Extended incubation of RH with enriched medium alters protein expression of key insulin signaling molecules.
(A) Representative immunoblot comparing cellular IRS1, IRS2, p85 and PTP1B levels at the time of seeding (0 d), and after 1 d, 2 d, and 3 d incubations in Waymouth’s medium ± 100 nM insulin. (B) Quantitation of protein expression changes 1 d, 2 d and 3 d relative to 0 d calculated as a percentage. Results from RH incubated without insulin are shown in black bars and RH incubated with insulin are shown in shaded bars. Results are means ± S.E.M., n = 4 independent rat liver experiments.
RH transitioning from IDAD sensitive to IDAD resistance, express elevated PTP1B which is known to be associated with resistance to insulin signaling in liver [24]. PTP1B is an ER-localized phosphatase [25], that negatively affects insulin signaling by dephosphorylation of phosphotyrosine residues (pY) on the insulin receptor (IR) and IRSs [24], thereby limiting downstream signaling cascades. Hepatic PTP1B expression is increased in vivo by fructose feeding [17], a regimen known to attenuate IDAD and stabilize hepatic B100 [16,17] leading to enhanced VLDL B100 secretion [16,17,26]. The increase in hepatic VLDL B100 production observed with fructose feeding is equivalent to that observed with in vivo wortmannin, a global PI3K inhibitor, providing further support for involvement of PI3Ks and protein tyrosine phosphatases in development of resistance to IDAD [16]. Current in vitro data in 3 d RH support the in vivo data with regards to the importance of PTP1B in development of IDAD resistance. We next examined McA cells to study VLDL metabolism and IDAD.
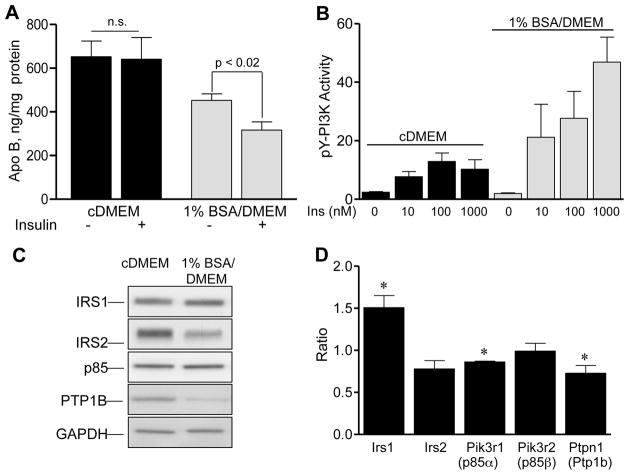
We compared McA cells incubated in serum-enriched complete medium (cDMEM) with McA cells incubated without serum in 1% BSA/DMEM to enhance insulin sensitivity. McA cells incubated in cDMEM secrete 30% more apo B-containing lipoproteins than those incubated without serum (652 ± 72 ng/mg cell protein, n = 8 versus 456 ± 30 ng/mg cell protein, n = 6, p < 0.02) (Fig. 2A). McA cells incubated in cDMEM are resistant to IDAD as insulin fails to suppress apo B secretion (4.1% ± 9.7%, n = 8, p = 0.18). In contrast, McA cells become sensitive to insulin following incubation in 1% BSA/DMEM and apo B secretion is reduced by 32.6 % ± 5.7 %, n = 6, p < 0.02). Results demonstrate an overall increase in secretion of apo B-containing lipoproteins by IDAD resistant McA cells incubated in cDMEM both in the presence and absence of insulin. Changes in IDAD sensitivity corresponded with differences in insulin-stimulated PI3K activity (Fig. 2B). While insulin stimulated PI3K activity in McA cells under both conditions, McA cells incubated in 1% BSA/DMEM demonstrated a much greater degree of insulin-stimulated PI3K activity. Comparing IDAD sensitive to IDAD resistant McA cells, insulin-stimulated PI3K activity was 2.5 ± 0.8 times greater with 10 nM insulin, 2.2 ± 0.2 times greater with 100 nM insulin, and 5.0 ± 0.7 times greater with 1000 nM insulin (n = 3, p < 0.05 at each insulin concentration). These data indicate that serum deprivation markedly increases insulin stimulated PI3K activation as well as restores IDAD.
Fig. 2. Characteristics of McA cells incubated in cDMEM versus 1% BSA/DMEM.
(A) Medium apo B concentration (ng/mg cell protein) in McA cells incubated in cDMEM (black bars) or in 1% BSA/DMEM (shaded bars) for 5 h. Results from incubations with and without 100 nM insulin are shown as means ± S.E.M., n = 8 for cDMEM and n = 6 for 1% BSA/DMEM incubations. n.s. no significant differences with insulin treatment were observed. (B) Insulin (5 min) stimulated PI3K activity of cellular pY immunoprecipitates of McA cells incubated in cDMEM (black bars) or in 1% BSA/DMEM (shaded bars). Results of the PI3 kinase assay (cpm x 10−3/μg protein) at each different insulin concentration are shown as means ± S.E.M. in n = 3 paired experiments. (C) Representative immunoblot comparing key insulin signaling molecules of McA cells incubated in cDMEM (left lane) or 1% BSA/DMEM (right lane). (D) Ratio of the relative abundance of mRNA transcripts present in McA cells incubated in 1% BSA/DMEM to McA cells incubated in cDMEM. Quantitations were performed by Q-RT-PCR and results are means ± S.E.M., n = 3 independent experiments. *Indicates differences in mRNA expression (p < 0.05).
We next addressed whether similar changes were observed in insulin signaling molecules in McA cells following the transition to IDAD resistance as occurred with RH. IDAD resistant McA cells expressed significantly elevated levels of IRS2 and PTP1B (Fig. 2C) similar to IDAD resistant RH. Following the transition to 1% BSA/DMEM, IRS2 was reduced by 33% ± 5.2% (n = 4, p < 0.05) and PTP1B was reduced by 36% ± 12.2% (n = 4, p < 0.05). Analysis of mRNA levels showed increases in Irs1 mRNA (p < 0.03), and decreases in p85α (Pik3r1, p < 0.001) and Ptp1b mRNAs (Ptpn1, p < 0.04). Ptp1b mRNA changes were consistent with PTP1B protein changes. To determine whether the increase in PTP1B observed in IDAD resistant McA cells played a role in the development of resistance, we treated McA cells incubated in cDMEM with RK682, an inhibitor of PTPs including PTP1B. RK682 had little effect alone on apo B secretion, however, insulin plus RK682 significantly reduced apo B secretion by 31% ± 9.7% (679 ± 116 ng/mg cell protein versus 501 ± 125, n = 4, p < 0.05). Overall, the data is consistent with a role for PTP1B in the development of IDAD resistance.
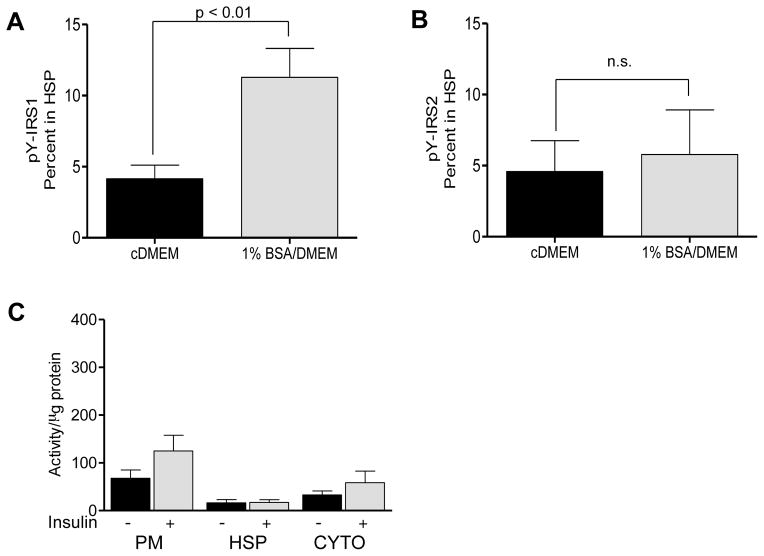
Our previous studies in RH demonstrated that IDAD correlates with the translocation of activated Class I PI3K to intracellular membranes [3]. To assess altered translocation, IDAD sensitive and IDAD resistant McA cells were incubated with insulin, and subcellular fractions were immunoprecipitated using an anti-pY antibody. This allowed for a direct comparison of changes in tyrosine phosphorylated IRS1 and IRS2 localized to intracellular membranes in response to insulin (Fig. 3AB). The percentage of pY-phosphorylated IRS1 present on microsomal membranes isolated in the high speed pellet (HSP) in response to insulin was 2.7 times greater in IDAD sensitive McA cells than in IDAD resistant McA cells (4.1 ± 0.97 versus 11.3 ± 2.0, n = 3, p < 0.01) (Fig. 3A). Corresponding analysis of pY-IRS2 showed no insulin-dependent changes in localization (Fig. 3B). As a control, we analyzed insulin stimulated PI3K activity of plasma membrane (PM), HSP and cytosol which indicated that changes in the HSP in IDAD resistant cells were not due to redistribution of activated PI3K to other fractions (Fig. 3C). These findings indicate that in IDAD sensitive McA cells, there is enhanced localization of pY-phosphorylated IRS1 on microsomal membranes with insulin similar to earlier reports in RH [3].
Fig. 3. Subcellular localization of pY phosphorylated IRS1 and IRS2 and PI3K activity following insulin treatment.
Localization of tyrosine phosphorylated IRS1 (A) and IRS2 (B) on microsomal membranes isolated in the high speed pellet (HSP) of McA cells incubated in cDMEM (black bars) or in 1% BSA/DMEM (shaded bars) following 15 min exposure to insulin. Results are means ± S.D., n = 3 independent experiments. (C) PI3K activity of anti-pY immunoprecipitates of subcellular fractions derived from McA cells incubated in cDMEM in the absence of insulin (black bars) or following exposure to insulin for 15 min (shaded bars). Results are mean activity (cpm x 10−3/μg protein) ± S.E.M., n = 4 independent experiments.
As shown in the current study, in IDAD resistant RH [20] and IDAD resistant McA cells, there is increased apo B secretion which is not subject to insulin-mediated suppression. IDAD resistant McA cells demonstrate reduced insulin-dependent PI3K activation, and reduced translocation of pY-IRS1 to microsomes containing ER which correlates with increased PTP1B expression. The importance of PTP1B activity in the development of IDAD resistance is strengthened by the demonstration that even in IDAD resistant McA cells, inhibition of phosphotyrosine phosphatases including PTP1B can restore IDAD. The physiological significance of IDAD resistance is that there are increased levels of hepatic VLDL secreted during the postprandial period which can compete with intestinal lipoproteins for catabolism, and lead to hypertriglyceridemia [27]. Future studies of pathways involved in IDAD will help identify relevant targets for regulation of VLDL secretion. In addition, short-term strategies to increase hepatic VLDL secretion might be developed that suppress IDAD, and favor triglyceride unloading to reduce hepatic steatosis. In summary, we have provided further support for the role of Class I PI3K in mediating IDAD, and its importance in involvement in IDAD resistance in liver. Although other factors are involved in IDAD including sortilin binding to apo B, autophagy and lysosomal degradation, the loss of signal generation and localization to intracellular membranes by Class I PI3K is likely to play a major role involved in IDAD resistance. Further studies will be necessary to define each step mechanistically from insulin activated PI3K, apo B lipoprotein binding to sortilin and downstream degradation through autophagy. Results presented in this study suggest resistance to IDAD takes place early in the proposed sequence of events resulting in IDAD.
Supplementary Material
Research highlights.
Enriched culture conditions increase apo B secretion by McA cells
Insulin induced Class I PI3K activity is blunted in McA cells under enriched conditions
IDAD sensitive McA cells demonstrate insulin-dependent pY-IRS1, but not pY-IRS2, translocation to intracellular membranes
RH and McA cells resistant to IDAD express increased PTP1B
Inhibition of protein tyrosine phosphatases including PTP1B is sufficient to restore IDAD in insulin resistant McA cells
Acknowledgments
This study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK100163).
Abbreviations
- Apo B
apolipoprotein B
- ER
endoplasmic reticulum
- IDAD
insulin-dependent apolipoprotein B degradation
- IRS1
insulin receptor substrate 1
- IRS2
insulin receptor substrate 2
- McA cells
McArdle RH7777 cells
- PI3K
phosphatidylinositide 3-kinase
- PIP3
phosphatidylinositide (3,4,5) triphosphate
- PTP1B
protein tyrosine phosphatase 1B
- RH
rat hepatocytes
- VLDL
very low density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 2.Sparks JD, Sparks CE. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem. 1990;265:8854–8862. [PubMed] [Google Scholar]
- 3.Phung TL, Roncone A, Jensen KL, Sparks CE, Sparks JD. Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J Biol Chem. 1997;272:30693–30702. doi: 10.1074/jbc.272.49.30693. [DOI] [PubMed] [Google Scholar]
- 4.Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42:833–842. doi: 10.2337/diab.42.6.833. [DOI] [PubMed] [Google Scholar]
- 5.Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40:454–462. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 6.Taskinen MR, Adiels M, Westerbacka J, Soderlund S, Kahri J, Lundbom N, Lundbom J, Hakkarainen A, Olofsson SO, Orho-Melander M, Boren J. Dual metabolic defects are required to produce hypertriglyceridemia in obese subjects. Arterioscler Thromb Vasc Biol. 2011;31:2144–2150. doi: 10.1161/ATVBAHA.111.224808. [DOI] [PubMed] [Google Scholar]
- 7.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 8.Sparks JD, Chamberlain JM, O’Dell C, Khatun I, Hussain MM, Sparks CE. Acute suppression of apo B secretion by insulin occurs independently of MTP. Biochem Biophys Res Commun. 2011;406:252–256. doi: 10.1016/j.bbrc.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirieac DV, Cianci J, Collins HL, Sparks JD, Sparks CE. Insulin suppression of VLDL apo B secretion is not mediated by the LDL receptor. Biochem Biophys Res Commun. 2002;297:134–137. doi: 10.1016/s0006-291x(02)02140-x. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain JM, O’Dell C, Sparks CE, Sparks JD. Insulin suppression of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting. Biochem Biophys Res Commun. 2013;430:66–71. doi: 10.1016/j.bbrc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strong A, Patel K, Rader DJ. Sortilin and lipoprotein metabolism: making sense out of complexity. Curr Opin Lipidol. 2014;25:350–357. doi: 10.1097/MOL.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparks JD, O’Dell C, Chamberlain JM, Sparks CE. Insulin-dependent apolipoprotein B degradation is mediated by autophagy and involves class I and class III phosphatidylinositide 3-kinases. Biochem Biophys Res Commun. 2013;435:616–620. doi: 10.1016/j.bbrc.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreo U, Guo L, Chirieac DV, Tuyama AC, Montenont E, Brodsky JL, Fisher EA. Insulin-stimulated degradation of apolipoprotein B100: roles of class II phosphatidylinositol-3-kinase and autophagy. PLoS One. 2013;8:e57590. doi: 10.1371/journal.pone.0057590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparks JD, Sparks CE. Obese Zucker (fa/fa) rats are resistant to insulin’s inhibitory effect on hepatic apo B secretion. Biochem Biophys Res Commun. 1994;205:417–422. doi: 10.1006/bbrc.1994.2681. [DOI] [PubMed] [Google Scholar]
- 15.Sparks JD, Sparks CE, Miller LL. Insulin effects on apolipoprotein B production by normal, diabetic and treated-diabetic rat liver and cultured rat hepatocytes. Biochem J. 1989;261:83–88. doi: 10.1042/bj2610083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirieac DV, Davidson NO, Sparks CE, Sparks JD. PI3-kinase activity modulates apo B available for hepatic VLDL production in apobec-1−/− mice. Am J Physiol. 2006;291:G382–388. doi: 10.1152/ajpgi.00472.2005. [DOI] [PubMed] [Google Scholar]
- 17.Taghibiglou C, Rashid-Kolvear F, Van Iderstine SC, Le-Tien H, Fantus IG, Lewis GF, Adeli K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem. 2002;277:793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- 18.Sparks JD, Cianci J, Jokinen J, Chen LS, Sparks CE. Interleukin-6 mediates hepatic hypersecretion of apolipoprotein B. Am J Physiol. 2010;299:G980–989. doi: 10.1152/ajpgi.00080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparks JD, Corsetti JP, Sparks CE. Liver regrowth and apolipoprotein B secretion by rat hepatocytes following partial hepatectomy. Metabolism. 1994;43:681–690. doi: 10.1016/0026-0495(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsson OG, Duerden JM, Bartlett SM, Sparks JD, Sparks CE, Gibbons GF. The role of pancreatic hormones in the regulation of lipid storage, oxidation and secretion in primary cultures of rat hepatocytes. Short-and long-term effects. Biochem J. 1992;281(Pt 2):381–386. doi: 10.1042/bj2810381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparks JD, Collins HL, Sabio I, Sowden MP, Smith HC, Cianci J, Sparks CE. Effects of fatty acids on apolipoprotein B secretion by McArdle RH-7777 rat hepatoma cells. Biochim Biophys Acta. 1997;1347:51–61. doi: 10.1016/s0005-2760(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 22.Sparks JD, Bolognino M, Trax PA, Sparks CE. The production and utility of monoclonal antibodies to rat apolipoprotein B lipoproteins. Atherosclerosis. 1986;61:205–211. doi: 10.1016/0021-9150(86)90139-5. [DOI] [PubMed] [Google Scholar]
- 23.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 24.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 25.Stuible M, Tremblay ML. In control at the ER: PTP1B and the down-regulation of RTKs by dephosphorylation and endocytosis. Trends Cell Biol. 2010;20:672–679. doi: 10.1016/j.tcb.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000;275:8416–8425. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 27.Sparks CE, Sparks JD. Hepatic postprandial transition and very low-density lipoprotein biogenesis. Curr Opin Lipidol. 2013;24:450–452. doi: 10.1097/MOL.0b013e3283654ed0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.