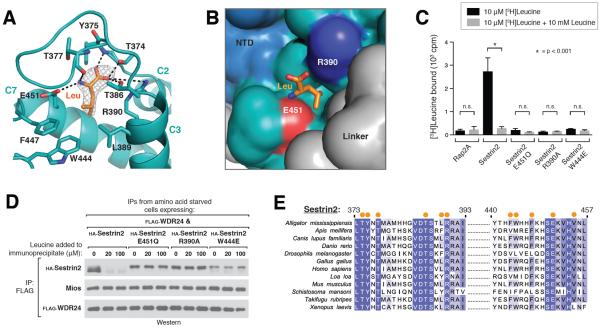
Figure 2. Recognition of leucine by Sestrin2.
A) Close-up view of the leucine binding pocket in Sestrin2, focusing on the bound leucine (shown in orange) together with its 2Fo-Fc electron density map calculated and contoured at 1.5σ from an omit map lacking leucine and all pocket residues. Predicted hydrogen bonds or salt-bridges are shown as black dashed lines. Helix numbers are labeled as in 1A.
B) Surface representation of leucine-bound Sestrin2, focusing on the leucine binding pocket. The bound leucine is represented as a stick model (orange). Residues 373–387 are omitted to allow visibility of the pocket. Residue Glu451, which contacts the amine of leucine is shown in red, and Arg390 which contacts the carboxyl of leucine is shown in blue. The domains of Sestrin2 are colored as in 1A.
C) Binding of the E451Q, R390A and W444E mutants of Sestrin2 to leucine. HA-immunoprecipitates prepared from HEK-293T cells transiently expressing indicated HA-tagged proteins were used in binding assays with [3H]Leucine. Binding was analyzed as in 1E.
D) Effect of leucine on the interactions of Sestrin2 E451Q, R390A or W444L with GATOR2. FLAG-immunoprecipitates were prepared from cells stably expressing FLAG-WDR24 and transiently expressing the indicated HA-tagged Sestrin2 constructs. The immunoprecipitates were treated with the indicated concentrations of leucine and analyzed by immunoblotting for the indicated proteins.
E) Multiple Sequence Alignment of Sestrin2 homologs from various organisms. Positions of residues contacting leucine are indicated with orange dots. Positions are colored white to blue according to increasing sequence identity.