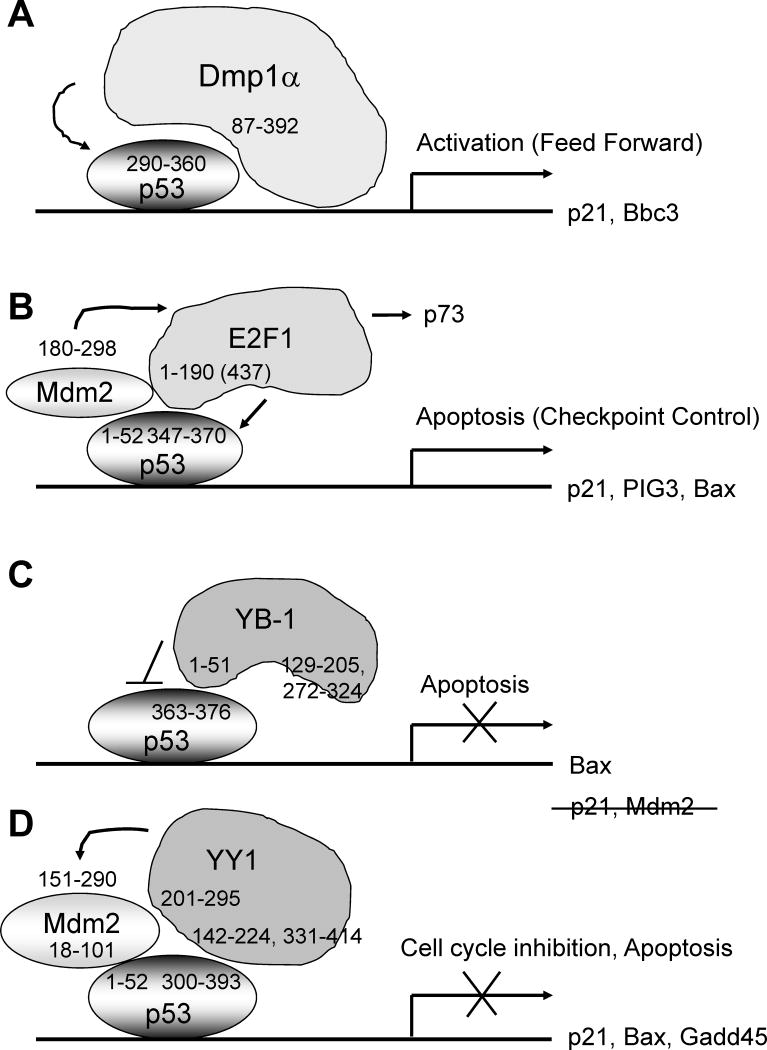
Figure 4. Schematic presentation of Dmp1α-p53, E2F1-p53/Mdm2, YB-1-p53, and YY1-p53/Hdm2 interactions and their biological consequences.
(A) Dmp1-p53 interaction. Dmp1α directly binds to the p53 C-terminus and neutralizes all the known functions for Mdm2 on p53 (31; arrow). This effect is mutually exclusive of DNA-binding of Dmp1α, and thus independent of Arf.
(B) E2F1-p53/Mdm2 interaction. E2F1 bind to p53 C-terminus through the N-terminal domain (major: a.a. 1–190, minor: a.a. 190–437; 47) or a.a. 1–108 (46). This interaction will provide a checkpoint mechanism to silence aberrant E2F1 signaling to apoptosis to prevent carcinogenesis (46). Mdm2 prolongs the half-life of E2F1 by inhibiting ubiquitination (50, arrow). Physical interaction of E2F1 and Mdm2 contributes to TAp73 transcriptional activity (horizontal arrow).
(C) YB-1-p53 interaction. YB-1 outside the cold-shock domain binds to p53 C-terminus to prevent the latter from inducing apoptosis genes (27). Thus YB-1 overexpression enhances tumorigenesis by accumulating cells that are not dying.
(D) YY1-p53/Hdm2 interaction. YY1 binds to both Hdm2 and p53, accelerates the polyubiquitination of p53 (arrow) to subvert the activities of p53. Since YY1 interacts with Hdm2 and p53 through different domains, they can form a ternary complex (30). This YY1-p53 interaction does not induce a checkpoint function to cancel the oncogenic activity of YY1.