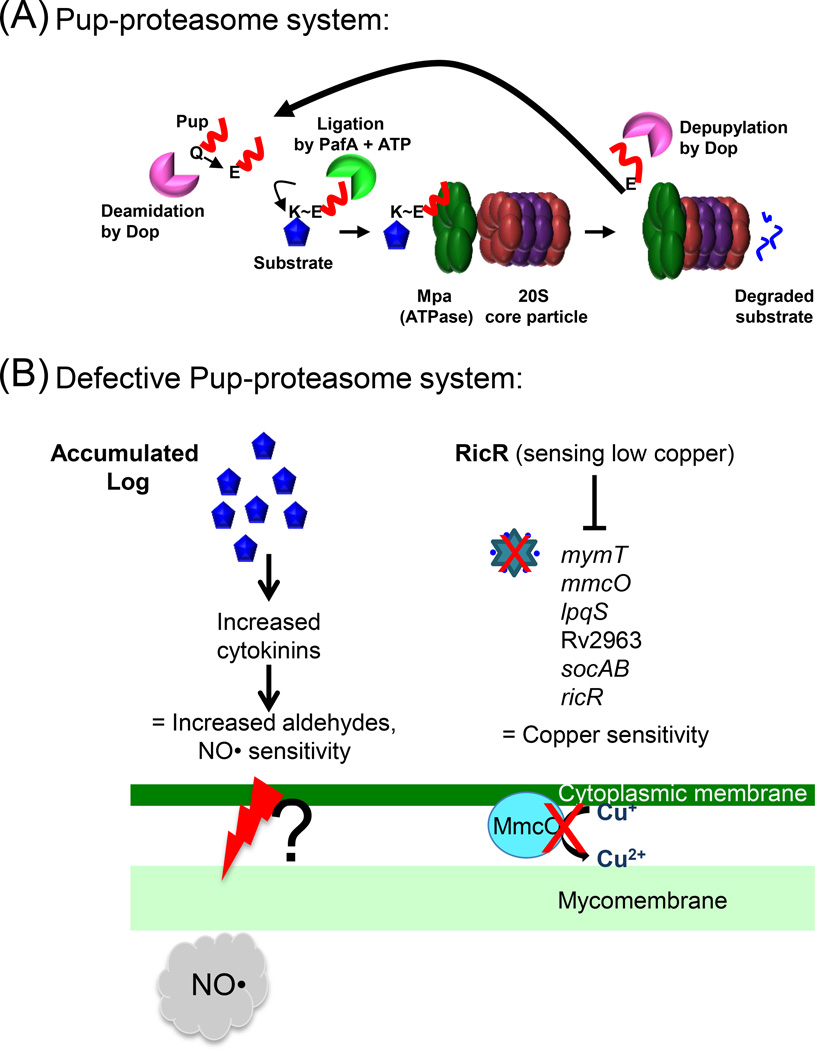
Figure 1. The Pup-proteasome System (PPS) of M. tuberculosis.
(A) ATP-dependent degradation by the proteasome. 20S core particle subunits are colored in red (α-subunit, PrcA) and purple (β-subunit, PrcB). Pup (red line) is an intrinsically disordered protein. In M. tuberculosis, the last amino acid of Pup is glutamine (Gln, Q), which must be deamidated on its side chain by Dop to convert it to glutamate (Glu, E). The Glu is the phosphorylated by PafA, making it prime for attack by the amino group on the side chain of a substrate lysine (Lys, K). Although Pup is largely disordered, it forms helices upon interacting with amino-terminal coiled-coil domains of Mpa. This interaction is essential for targeting pupylated proteins for degradation. In M. tuberculosis, Pup appears to be recycled by Dop in an Mpa-dependent manner, suggesting depupylation can occur just before proteins are threaded into the proteasome core particle. See text for additional details and references. (B) Disrupted proteasomal degradation results in several dysregulated pathways. Left: Accumulation of the enzyme Lonely Guy (Rv1205, Log) results in the hyperproduction of cytokinins, which can break down into aldehydes. Aldehydes synergize with NO by an unknown mechanism to kill mycobacteria. A mechanism of aldehyde-mediated toxicity might include damage (as represented by the red lightening bolt) to the mycobacterial membranes. This damage may be exacerbated in the presence of host-produced NO. Right: The RicR regulon is partially repressed in mpa and pafA mutants, leading to reduced copper (Cu) resistance. Red Xs indicate loss of MymT (Cu metallothionein) and MmcO (mycobacterial multi-copper oxidase) activities.