Abstract
Previously characterized nicotinic acetylcholine receptor (nAChR) autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE)-associated mutations are found in α2, α4 and β2 subunit transmembrane (TM) domains. They predominantly increase ACh potency and, for β2-subunit mutants, increase macroscopic currents. Two recently-identified mutations, α4(R336H) and β2(V337G), located in the intracellular cytoplasmic loop (C2) have been associated with non-familial NFLE. Effects of these mutations on α4β2-nAChR function and expression were studied for the first time, using two-electrode voltage clamp recordings in Xenopus laevis oocytes. Biased-ratio preparations elucidated the mutations’ effects at alternate isoforms: high-sensitivity [HS; (α4)2(β2)3] or low-sensitivity [LS; (α4)3(β2)2] via 1:10 or 30:1 [α4:β2] cRNA injection ratios, respectively. An unbiased (1:1 [α4:β2] cRNA) injection ratio was also used to study potential shifts in isoform expression. α4(R336H)-containing receptors showed significant increases in maximal ACh-induced currents (Imax) in all preparations (140% increase compared to wild type control). β2(V337G)-containing receptors significantly increased Imax in the LS-favoring preparation (20% increase compared to control). Expression of either mutation consistently produced enrichment of HS-isoform expression in all preparations. α4β2-nAChR harboring either NFLE mutant subunit showed unchanged ACh, sazetidine-A, nicotine, cytisine and mecamylamine potency. However, both mutant subunits enhanced partial agonist efficacies in the LS-biased preparation. Using β2-subunit-specific [125I]mAb 295 immunolabeling, nAChR cell-surface expression was determined. Antibody binding studies revealed that the β2(V337G) mutation tended to reduce cell-surface expression, and function per receptor was significantly increased by either NFLE mutant subunit in HS-favoring preparations. These findings identify both common and differing features between TM- and C2- domain AD/NFLE-associated mutations. As we discuss, the shared features may be particularly salient to AD/NFLE etiology.
Keywords: nicotinic acetylcholine receptor, nocturnal frontal lobe epilepsy, alpha4beta2 isoform, cytoplasmic loop
1. Introduction
1.1 nAChR subunit mutations linked to nocturnal frontal lobe epilepsy
Epilepsy is the most common neurological disorder, affecting roughly 1% of the population (Hauser et al., 1993; Leonardi and Ustun, 2002). Monogenic epilepsies, including nocturnal frontal lobe epilepsy (NFLE), represent 39–59% of all epilepsies (Motamedi and Lesser, 2002). NFLE is a group of familial (autosomal dominant [ADNFLE]) and sporadic disorders that are alike in their clinical seizure characteristics (dystonic posturing, rapid uncoordinated movements and vocalization), suggesting a shared the genetic basis for the nocturnal seizures. ADNFLE is a partial epilepsy inherited with a penetrance rate as high as 90% (Steinlein et al., 2012b). This disorder does not show a tendency of spontaneous remission and in nearly a third of all cases seizures are resistant to antiepileptic drug treatment (Provini et al., 1999). An enhanced understanding of disease-related mutation effects is necessary to develop better treatments for individuals with ADNFLE/NFLE.
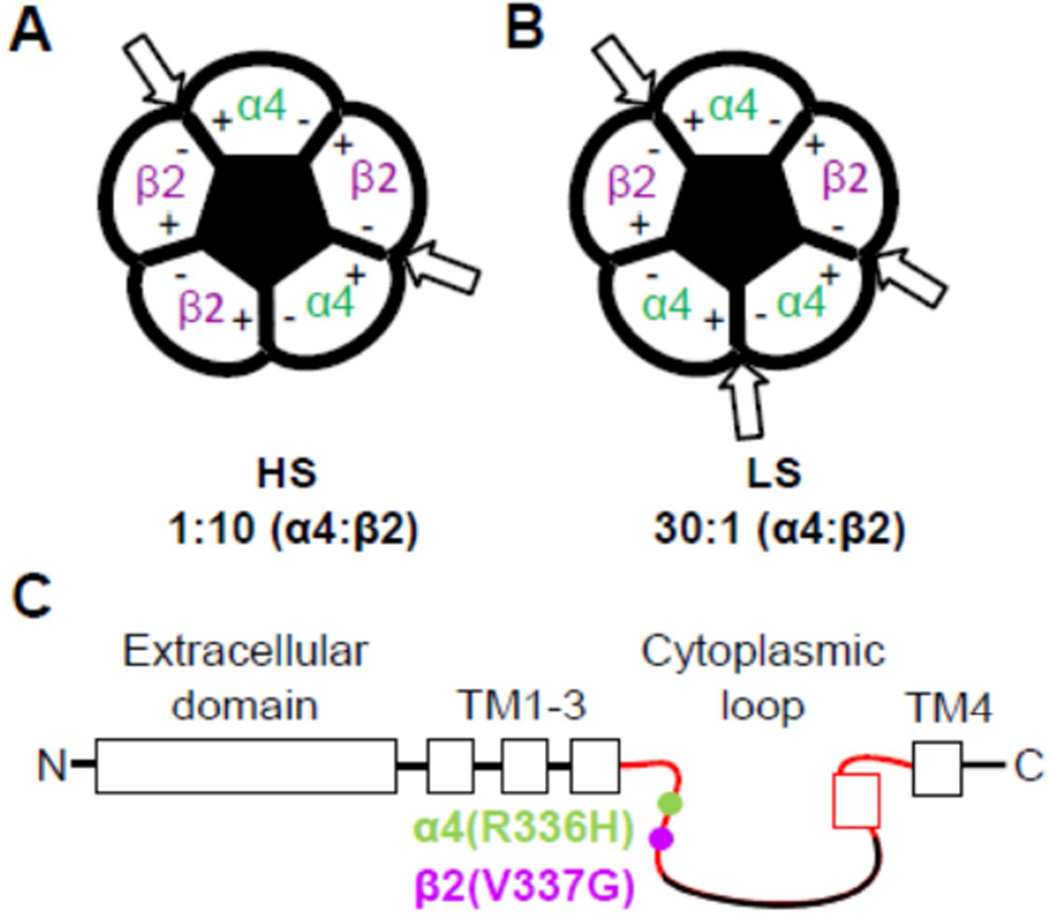
ADNFLE was the first human epilepsy where particular mutations in the subunits of nicotinic acetylcholine receptors (nAChR) were identified (Phillips et al., 1995; Scheffer et al., 1995; Scheffer et al., 1994; Steinlein et al., 1995). nAChR are cholinergic pentameric ligand gated ion channels. α4β2*-nAChR are the most-prevalent subtype expressed in the central nervous system (Taly et al., 2009) (* denotes the possible presence of other subunits (Lukas et al., 1999)), and are highly concentrated in the thalamocortical network (Lambe et al., 2003), a brain system implicated in epilepsy (Picard et al., 2006). α4β2-nAChR exist as two isoforms with distinct stoichiometries and high- or low- sensitivity to nicotinic agonists [HS (α4)2(β2)3 or LS (α4)3(β2)2, respectively] (Figure 1A and B) (Briggs et al., 2006; Eaton et al., 2014; Mazzaferro et al., 2011; Moroni and Bermudez, 2006; Nelson et al., 2003; Tapia et al., 2007). Expression of either isoform can be encouraged using different α4:β2 subunit ratios in heterologous systems (Figure 1A and B) (Zwart and Vijverberg, 1998) that mimic the natural isoforms found in the mammalian brain (Gotti et al., 2008; Marks et al., 1999).
Figure 1. (1-column width). Schematic representations of α4β2 nAChR HS- and LS-isoforms and location of the C2 NFLE mutations.
Agonist binding sites, as indicated by the arrows, are located between the principal (+) faces of the α4 subunits and the complementary (−) faces of the β2 subunits. An additional, LS-isoform-specific, agonist binding site is found at the α4(+)/α4(−) interface. A) Preferential expression of the HS-isoform was achieved by injecting a higher ratio of the β2 subunit cRNA (1 ng of α4 : 10 ng of β2). B) Expression of the LS-isoform was encouraged by using a 30 ng of α4 : 1 ng of β2 cRNA injection ratio. C) Schematic showing the location of the cytoplasmic loop (C2) NFLE subunit mutations, near the transmembrane (TM) 3 domain. Sites of the polymorphisms are separated by nine amino acid residues. Regions of the C2 loop that are highly conserved between subunits are highlighted in red, including the MA-helix (indicated by the red box). Numbering based on NCBI reference sequence NP_000735.1 (α4) and NP_000739.1 (β2).
1.2 Functional effects of previously-characterized NFLE-linked, transmembrane-domain nAChR subunit mutations
Eight mutations located in the second or third transmembrane (TM) domains of the α4 or β2 nAChR subunits have been linked with ADNFLE (Bertrand et al., 2005; De Fusco et al., 2000; Hirose et al., 1999; Steinlein, 2004, 2010; Steinlein et al., 1997; Steinlein et al., 1995). Electrophysiological examination of these TM domain mutations’ effect on function in heterologous expression systems has revealed a mixture of properties. The predominant outcomes were increased ACh potency and (often) efficacy (see Discussion for details).
1.3 Characterization of recently identified NFLE-linked nAChR subunit mutations located in the major intracellular cytoplasmic loop domain will likely provide further disease insights
Recently, two mutations were identified, but not functionally evaluated, in the long second cytoplasmic loop (C2) that links TM helices 3 and 4 of the α4- or β2- nAChR subunits (close to the TM3 domain; Figure 1C). These were found in individuals with NFLE, and were not found in control subjects (Chen et al., 2009; Liu et al., 2011). These individuals experienced nocturnal seizures that were comparable to ADNLFE seizures. The first question we wished to address was whether these newly identified mutants have any measureable effects on α4β2-nAChR function and, if so, whether such effects resemble overall those produced by established ADNFLE-linked nAChR subunit mutants. Without functional consequences, it is highly unlikely that the novel mutants could be associated with the symptoms seen in the initial discovery papers (Chen et al., 2009; Liu et al., 2011). In addition, the α4(R336H) (formerly mislabeled as α4(R308H)) (Chen et al., 2009) and β2(V337G) (Liu et al., 2011) mutant subunits are the first NFLE-associated mutations found within the C2 region. The C2 nAChR subunit domain is relatively under-studied, but has been associated with regulating channel conductance, assembly and cell-surface expression (Hales et al., 2006; Kracun et al., 2008; Kuo et al., 2005; Tsetlin et al., 2011). Given the C2 mutants’ novel location, we hypothesized that their incorporation may alter HS- and LS- α4β2-isoform expression and function somewhat differently, when compared in detail, to TM ADNFLE mutants.
Features conserved across both classes of mutants may be particularly relevant to causing AD/NFLE. Accordingly, the present study is intended to bring functional characterization of C2 mutant subunits’ effects on α4β2-nAChR function to the same level as that of the ADNFLE TM-mutant subunits. We also have added, unusually for ADNFLE-associated mutations, characterization of the α4(R336H) and β2(V337G) subunits’ effects on HS- and LS-α4β2-isoform function and cell-surface expression. Significant functional- and surface-expression-level effects were seen, with both contrasts and points of similarity to outcomes produced by ADNLFE TM-domain mutant subunits. In addition to highlighting the similar features as likely being particularly salient to NFLE causation, our study demonstrates important roles for the relatively-conserved cytoplasmic loop sequence near to TM3 in mediating cell-surface expression, isoform assembly and per-receptor function.
2. Materials and Methods
2.1 Reagents
Dihydro-β-erythroidine hydrobromide (DHβE) and mecamylamine hydrochloride were purchased from Tocris (Bristol, UK). Sazetidine-A [6-(5-(((S)-azetidin-2-yl)methoxy)pyridine-3-yl)hex-5-yn-1-ol] (Xiao et al., 2006), also known as AMOP-H-OH, was a generous gift from Dr. Alan P. Kozikowski (University of Illinois, Chicago, IL). [125I]mAb 295 was provided by Dr. Jon M. Lindstrom (University of Pennsylvania, Philadelphia, PA). All other reagents and pharmacological ligands (acetylcholine chloride (ACh), (−)-nicotine hydrogen tartrate salt and cytisine) were purchased from Sigma (St. Louis, MO) unless otherwise specified. Fresh solution stocks were made daily and diluted as required.
2.2 DNA constructs and cRNA synthesis
The cDNA sequences for human wild type α4 (NCBI Reference Sequence: NM_000744.5), wild type β2 (NCBI Reference Sequence: NM_000748.2), α4(R336H) (Chen et al., 2009) and β2(V337G) (Liu et al., 2011) were used to synthesize full-length cDNA for each subunit (Life Technologies, Grand Island, NY). All constructs were fully sequenced and confirmed to be identical to the published sequences for each subunit. Each nAChR subunit cDNA was removed from the pMA shuttle vector using Not I and Xba I restriction enzymes (New England Biolabs, Ipswich, MA) and ligated into the pCI mammalian expression vector (Promega Madison, WI) using T4 DNA ligase (Promega, Madison, WI). The constructs were transformed into NEB 5-α competent E. coli cells (New England Biolabs, Ipswich, MA) for larger-scale production of cDNA. DNA was isolated using QIAprep Spin Miniprep kits (Qiagen, Valencia, CA). To prepare for cRNA synthesis, cDNA clones of the α4, α4(R336H), β2 and β2(V337G) subunits were linearized with the restriction enzyme Swa I and treated with proteinase K (30min at 50°C), then purified using Qiagen’s PCR clean-up kit. cRNAs were transcribed using the T7 mMESSAGE mMACHINE™ High Yield Capped RNA Transcription Kit (Ambion, Austin, TX). cRNA purity was confirmed on a 1% agarose gel and the final product was sub-aliquoted and stored at −80°C.
2.3 Oocyte preparation and cRNA injection
All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available. Xenopus laevis harvested and de-folliculated stage V oocytes were purchased from EcoCyte Bioscience (Austin, TX). cRNA was injected into Xenopus oocytes either in an equal (unbiased) ratio of α4:β2 subunits or biased ratios. Unbiased expression of both isoforms was accomplished by using a 1:1 cRNA injection ratio of α4 and β2 subunit cRNAs (1 ng of α4 : 1 ng of β2). Expression of predominantly either high (HS) or low (LS) ACh sensitivity α4β2 receptors was enhanced by injection of different cRNA ratios (1 ng of α4 : 10 ng of β2 for HS and 30 ng of α4 : 1 ng of β2 for LS). Please note that expression ratios referred to throughout the manuscript are reported with the ratio of α4 being listed first followed by the β2 subunit (e.g. 1:1 [α4:β2]). LS α4β2-nAChR expressed either via biased loose subunit cRNA injection ratios [>4:1 α4:β2] or as LS concatenated receptors display an intrinsic biphasic ACh concentration-response profile having high- and low- ACh potency phases (Eaton et al., 2014; Harpsoe et al., 2011). At the high-ACh potency phase, smaller currents were recorded compared to the low-ACh potency phase in LS-isoform (Eaton et al., 2014; Harpsoe et al., 2011). For this study, nAChR were expressed via loose subunits rather than concatenated receptors to permit the examination of possible effects of the C2 NFLE mutations on HS- versus LS- isoform expression ratios, as noted previously for TM-located NFLE mutations (Son et al., 2009). In all cases, 81 nl of cRNA was injected into each oocyte by impalement via a pulled micropipette with an outer diameter of ~40 µm. Oocytes were incubated at 13°C for at least 72h prior to re cording.
2.4 Two-electrode voltage clamp (TEVC) electrophysiology
At least three days after cRNA injection, Xenopus oocytes expressing either α4β2-, α4R336Hβ2- or α4β2V337G- nAChR were voltage-clamped at −70 mV with an Axoclamp 900A amplifier (Molecular Devices, Sunnyvale, CA). Data acquisition and analysis were performed using pClamp 10.2 software (Molecular Devices, LLC, Sunnyvale, CA). Recordings were sampled using a 10 kHz low-pass Bessel filter and 40 Hz high-pass filtered to suppress DC offset. Recording electrodes were pulled from thin wall capillary glass and filled with 3M KCl. Electrode resistance ranged from 0.5 – 10 MΩ. Oocytes with leak currents >100 nA were not used for experimental recordings.
To investigate if receptor pharmacology was altered by incorporation of the C2 NFLE mutations, concentration-response data were collected using several pharmacological ligands. Half-log concentration ranges of ACh (0.001–3000 µM), nicotine (0.0003–1000 µM), cytisine (0.001–1000 µM), sazetidine-A (0.0001–10 µM), DHβE (0.001–300 µM) and mecamylamine (0.0003–100 µM) were applied to clamped oocytes using a 16 channel, gravity-fed, perfusion system with automated valve control (AutoMate Scientific, Inc; Berkeley, CA). The antagonists DHβE and mecamylamine were co-applied with the ACh EC90 concentration (30 µM HS, 200 µM LS). All solutions were made in OR2 recording buffer (92.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2·6H2O, 1 mM CaCl2·2H2O, 5 mM HEPES, pH to 7.5 using NaOH). Atropine sulfate (1.5 µM) was added to all recording solutions to block any potential muscarinic responses. All ligands tested were applied to the receptor-expressing oocyte using 1s valve openings (henceforth referred to as “1 s applications”), at a flow rate of 4ml/min. As described in our previous publication (Eaton et al., 2014), post-valve tubing lengths are minimized and a custom manifold was used to reduce dead volume. These optimizations optimize solution exchange at the oocyte and result in a peak application time of approximately 0.8s (defined as ≥90% of full concentration). Application rise times to 90% of full concentration are ≈0.2 s and washout requires ≈0.4s (Eaton et al., 2014). A recovery period of 60s between applications was used for all tested ligands with the exception of sazetidine-A. Sazetidine-A is a ligand with very high affinity and thus a slower dissociation rate, and required 85s for complete recovery of responses between applications.
In addition to concentration-response curves, ACh-induced currents (Imax) were measured at a maximally effective concentration (300 µM for 1:1 and 1:10, and 1 mM for the 30:1 α4:β2 preparations), and used as a gauge of macroscopic receptor function across oocyte incubation time following cRNA injection. All responses were normalized to maximum α4β2 wild type peak current responses (day 10 for 1:1 and 1:10 preparations and day 7 for 30:1 preparation). The maximal peak current induced by 300 nM sazetidine-A was also measured and used to determine the proportional expression of the HS- and LS- isoforms in each preparation. We previously measured the sazetidine-A efficacy to be 12 ± 2% on LS- and 100% efficacious on HS- concatenated receptors (Eaton et al., 2014). Additionally, sazetidine-A efficacy has been shown to be similar for α4β2 receptors expressed via loose and concatenated receptor techniques (Carbone et al., 2009). Given the above, we defined ACh and sazetidine-A peak current (peak I) responses in terms of contribution from the HS- and LS- isoforms:
| (1) |
and
| (2) |
We then defined x to be the ratio of the sazetidine-A and ACh peak I responses:
| (3) |
By re-arranging equation 3 and expressing the resulting equation in terms of HS and LS using equations 1 and 2, we calculated the response ratio of LS to HS in each preparation at the different time points post cRNA injection:
| (4) |
To simplify equation 4, we renamed the resulting equation of the LS to HS ratio to A and solved for LS:
| (5) |
Because we know that activation of both the HS- and LS- isoforms contribute to the ACh peak response and the response from both isoforms results in 100% of the ACh induced response, we determined that the individual HS and LS proportional responses would sum to 1:
| (6) |
We next calculated the proportional expression of the LS-isoform in terms of A (by using equation 5) and LS (using equation 6: HS = 1 – LS):
| (7) |
Given that the HS and LS proportions will sum to 1, we were then able to solve for the proportional expression of the HS-isoform (equation 6: HS = 1 - LS).
2.5 [125I]mAb 295 immunolabeling of cell-surface β2 subunits
The C2-domain NFLE mutations could potentially alter the expression of α4β2-nAChR on the surface of Xenopus laevis oocytes. To determine this, cell-surface nAChR expression levels were measured using antibody-binding assays. Total α4β2-nAChR function and the proportion expressed as HS- or LS- α4β2-nAChR isoforms were measured for individual oocytes using TEVC electrophysiology (as just described). [125I]mAb 295 labeling was then used to measure nAChR expression on the surface of the same oocytes. mAb 295 is a rat monoclonal antibody that was originally produced against purified chicken-brain nAChRs. It has been shown to recognize human, bovine and rodent nAChR β2 subunits in native form with great specificity (Lai et al., 2005; Whiteaker et al., 2006; Whiting and Lindstrom, 1988). This well-established method has formerly been described to compare β2*-nAChR function and cell-surface expression with high sensitivity (Eaton et al., 2014; Kuryatov and Lindstrom, 2011). Oocytes were incubated for 3h in OR2 buffer supplemented with heat-inactivated normal fetal bovine serum (10%; to reduce nonspecific binding) (Gibco Life technologies, Grand Island, NY) and a saturating concentration (2 nM (Whiteaker et al., 2009)) of [125I]mAb 295. Unbound and nonspecifically bound [125I]mAb 295 were removed via three, 2min washes with ice-cold OR2 buffer. Residual nonspecific binding was determined by incubating non-injected control oocytes in the same assay. Nonspecific binding was subtracted from the total binding of each tested oocyte to calculate the specific binding. Specific cell-surface binding of [125I]mAb 295 was converted to nAChR surface expression using the specific activity of the radiolabeled antibody, proportional expression of the HS- and LS- isoforms (as described in Section 2.4) and accounting for two or three β2 binding sites for either LS ([α4]3[β2]2 stoichiometry) or HS ([α4]2[β2]3 stoichiometry) isoforms, respectively.
2.6 Data Analysis
pEC50 (negative log10EC50 value), pIC50 (negative log10IC50 value), Hill slopes (nH) and peak current amplitude (Imax) values were determined from individual oocytes. All experiments were conducted on at least two batches of cRNA synthesis and three oocyte isolations. For each set of experiments, the number of experimental replicates are indicted by large N followed by the number individual of oocytes are represented by small n throughout the manuscript. Concentration-response profiles were calculated using non-linear curve fitting in GraphPad Prism 5.03 Software (La Jolla, CA). Unconstrained monophasic sigmoidal or constrained (nH = 1) biphasic logistic equations were used to fit all parameters. A sum-of-squares F-test was used to verify when data were better fit by the biphasic rather than monophasic model. Data were analyzed using Student’s t test to compare pairs of groups. Two-way ANOVA with a Bonferroni post hoc test or one-way ANOVA and Tukey’s multiple comparison test were used to evaluate the means of three or more groups. Statistical analyses were also performed using GraphPad Prism 5.03.
3. Results
3.1 C2 NFLE mutant subunits have no effect on ACh potency
As noted in the Introduction, a predominant effect of TM-domain NFLE mutations in α4 or β2 nAChR subunits is to increase ACh potency. To test for potential effects of the C2 NFLE mutations (α4(R336H) and β2(V337G)) on ACh potency, we expressed wild type and mutant subunits in Xenopus laevis oocytes using 1:10 and 30:1 α4:β2 cRNA injection ratios to enforce the biased expression of either the HS- or LS- α4β2-nAChR isoform. A 1:1 [α4:β2] ratio was also used to facilitate the observation of potential changes in expression of the α4β2-nAChR HS- versus LS- isoforms induced by the mutant subunits.
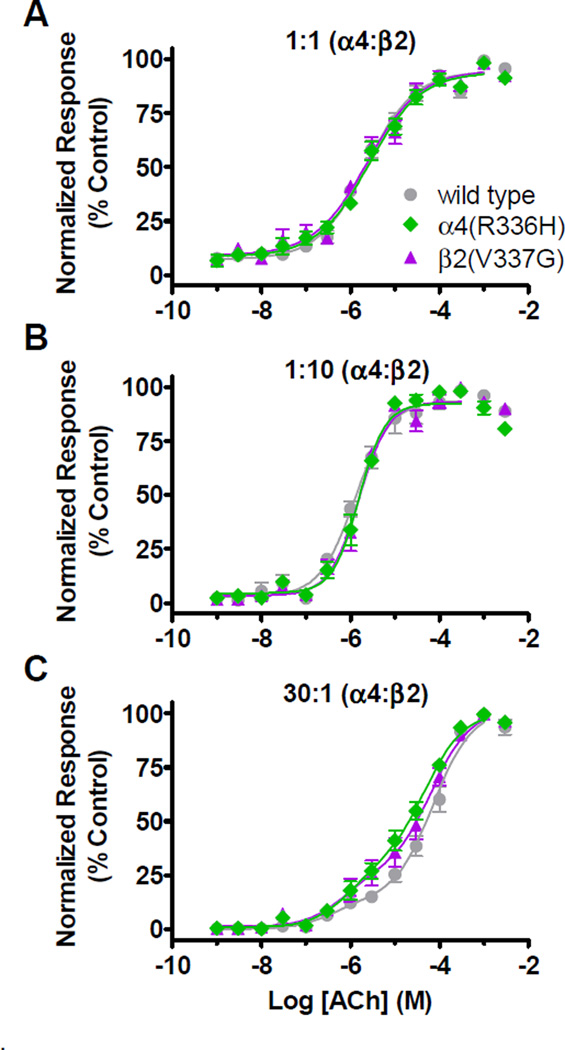
Expression of wild type receptors in the 1:10 and 30:1 [α4:β2] biased expression systems revealed that, as expected, the pEC50 values differed between the two preparations (Figures 2B, 2C and Table 1). The 1:10 wild type preparation produced receptors that had a high sensitivity to ACh (EC50 approximately 1.3 µM). The 30:1 preparation yielded concentration-response profiles that were best fit using a biphasic equation rather than a single sigmoidal fit. Wild type α4β2-nAChR expressed in the 30:1 preparation had smaller high-sensitivity (HS) (EC50_HS approximately 0.40 µM) and larger low-sensitivity (LS) (EC50_LS approximately 72 µM) phases of function (Figure 2C and Table 1). The wild type 30:1 preparation HS-phase potency was similar to the ACh potency in the 1:10 preparation. In contrast, 30:1 wild type receptors’ ACh potency for the LS-phase was significantly lower than that observed for the wild type receptor 1:10 preparation (Table 1). The presence of a small proportion of HS-like activity is an intrinsic property of (α4)3(β2)2-stoichiometry (LS-isoform) nAChR populations, and the observed EC50 values are consistent with previous investigations studying α4β2 HS- and LS- isoforms expressed in oocytes using unlinked and/or concatenated subunits (Eaton et al., 2014; Harpsoe et al., 2011; Moroni and Bermudez, 2006). ACh concentration-response profiles showed that wild type subunit-containing receptors expressed via the 1:1 [α4:β2] preparation were best fit with a monophasic model. However, this produced pEC50 values that indicated slightly, but significantly, lower-potency than those measured in the 1:10 preparation (Figures 2A, 2B and Table 1). The 1:1 [α4:β2] data were also best fit with a shallower nH. These findings suggest that a small population of the LS-isoform may be expressed in addition to the HS-isoform, even if this cannot be resolved reliably in the concentration-response data (Table 1). These results are again consistent with the literature. Previous studies have found that 1:1 injections can produce a variety of outcomes spanning α4β2-nAChR populations with either predominantly-HS (Figl et al., 1998; Weiland et al., 1996), primarily-LS (Son et al., 2009; Zwart and Vijverberg, 1998), or mixed nAChR populations with distinctly biphasic, ACh concentration-response curves (Bertrand et al., 2005; Bertrand et al., 2002; Moroni et al., 2006a; Steinlein et al., 2012a).
Figure 2. (1 column width). C2 NFLE mutant subunits expressed in Xenopus oocyte preparations had minimal effects on α4β2-nAChR ACh concentration-response profiles.
Xenopus oocytes injected with wild type or mutant cRNA in unbiased (1:1 [α4:β2]) or biased (1:10 or 30:1 [α4:β2]) ratios were exposed to 1s applications of increasing concentrations of ACh on day 3 post cRNA injections. A and B) ACh concentration-response curves showed that the pEC50 values for the 1:1 and 1:10 preparations were similar between wild type and C2 NFLE mutant containing receptors. However, the pEC50 and nH values were slightly, but significantly, different between the preparations suggesting that the 1:1 preparation may contain a mixture of the α4β2-isoforms (Table 1). C) ACh concentration-response data collected using the 30:1 cRNA injection preparation were best fit using a biphasic rather than a single-phase sigmoidal equation; they have distinct HS- and LS-phases (see Table 1). No effect of the C2 NFLE mutations was seen on pEC50 values; however, a tendency was seen for the C2 NFLE mutations to enhance the HS-phase fraction. pEC50, Hill slopes (nH) and HS-phase fraction values are reported in Table 1, together with details of the statistical analysis. Points are the mean ± S.E.M. (N = 2, n = 4 – 13), and % control represents the ACh-induced response normalized to the maximum observed ACh induced current.
Table 1.
NFLE mutant subunit effects on ACh potency and Hill slope (nH) values in 1:1, 1:10 and 30:1 [α4:β2] nAChR expression preparations.
| Subunits | Ratio (α4:β2) |
N (n) | pEC50_HS ± SEM |
nH_HS ± SEM |
pEC50_LS ± SEM |
% HS- fractio |
|||
|---|---|---|---|---|---|---|---|---|---|
| α4:β2 | 1:1 | 2 (13) | 5.64 ± 0.05 |
|
0.80 ± 0.07 |
|
N/A | 100 | |
| α4(R336H)β2 | 1:1 | 2 (11) | 5.58 ± 0.06 | 0.75 ± 0.07 | N/A | 100 | |||
| α4:β2(V337G) | 1:1 | 2 (13) | 5.62 ± 0.07 | 0.74 ± 0.07 | N/A | 100 | |||
| α4:β2 | 1:10 | 2 (6) | 5.90 ± 0.05 | 1.09 ± 0.13 | N/A | 100 | |||
| α4(R336H)β2 | 1:10 | 2 (6) | 5.81 ± 0.04 | 1.47 ± 0.20 | N/A | 100 | |||
| α4:β2(V337G) | 1:10 | 2 (4) | 5.81 ± 0.05 | 1.29 ± 0.18 | N/A | 100 | |||
| α4:β2 | 30:1 | 2 (6) | 6.40 ± 0.40 | Fixed = 1 | 4.14 ± 0.07 |
|
14 ± 3 | ||
| α4(R336H)β2 | 30:1 | 2 (7) | 6.03 ± 0.22 | Fixed = 1 | 4.30 ± 0.10 | 30 ± 5 | |||
| α4:β2(V337G) | 30:1 | 2 (5) | 6.15 ± 0.31 | Fixed = 1 | 4.17 ± 0.12 | 26 ± 6 |
Wild type and mutant nAChR subunits were expressed in Xenopus oocytes using 1:1, 1:10 or 30:1 [α4:β2] cRNA injection ratios. Experimental ACh concentration-response curve analysis details can be found in the Materials and Methods and Figure 2 legend. Data collected from the 1:1 or 1:10 preparations were best fit using a monophasic logistic equation. The pEC50_HS values of the 1:1 preparation were significantly different when compared to the 1:10 cRNA injection preparations, but no effect of the NFLE mutant subunits was found (two-way ANOVA with Bonferroni multiple comparisons post hoc test: receptor subunits F2,6= 0.89, P = 0.46; cRNA injection preparation F1,6= 23.67, P = 0.0028; interaction receptor subunits x cRNA injection preparation F2,6= 0.22, P = 0.81). There was a significant difference found in the nH values between the 1:1 and 1:10 preparations, but the effect was not caused by the NFLE mutant subunits (two-way ANOVA with Bonferroni multiple comparisons post hoc test: receptor subunits F2,6= 0.73, P = 0.52; cRNA injection preparation F1,6= 23.06, P = 0.0030; interaction receptor subunits x cRNA injection preparation F2,6= 1.32, P = 0.34). These results suggest that the 1:1 preparation may contain a mixture of HS- and LS- isoforms. The 30:1 preparation data was best fit using a biphasic logistic equation with nH values fixed to 1 (30:1 Comparison of Fits test results, where P < 0.05 resulted in rejection of the data being best fit using a monophasic logistic equation: α4β2 F1,78 = 15.16, P = 0.0002; α4(R336H)β2 F1,93 = 9.84, P = 0.0023; α4β2(V337G) F1,93 = 7.61, P = 0.0075). The 1:10 and 30:1 preparations ACh pEC50_HS values were not significantly different between mutant and wild type receptor subunits and cRNA injection preparations (two-way ANOVA with Bonferroni multiple comparisons post hoc test: receptor subunits F2,6= 0.55, P = 0.61; cRNA injection preparations F1,6= 3.71, P = 0.10; interaction receptor subunits x cRNA injection preparation F2,6= 0.19, P = 0.83). However, the 30:1 preparation LS-phase potency (pEC50_LS) values were significantly less when compared to the ACh potency of the 1:10 preparation, but no effect of the C2 NFLE mutant subunits was found (two-way ANOVA with Bonferroni multiple comparisons post hoc test: receptor subunit F2,6= 0.38, P = 0.70; cRNA injection preparation F1,6= 636.7, P < 0.0001; interaction receptor subunit x cRNA injection preparation F2,6= 1.29, P = 0.34). In the 30:1 preparation, a trend was seen for the C2 NFLE mutant subunits to enhance the HS-fraction compared to wild type receptors but the effect was not significant (one-way ANOVA with Tukey’s post hoc test: F2,3 = 2.97, P = 0.19).
All values reported in the table are the mean ± S.E.M. Significant changes are noted as follows:
P < 0.05;
P < 0.01;
P < 0.0001 (two-way ANOVA with Bonferroni post hoc analysis).
ACh potency was unchanged between α4β2-nAChR expressed using wild-type subunits and those incorporating either of the C2 NFLE mutations. This was true in each case, across the 1:1, 1:10 or 30:1 injection ratios (Figures 2A – C and Table 1). However, in the 30:1 [α4:β2] preparations, the amount of HS-phase function within the biphasic concentration-response curves tended to increase, even as the measured EC50 values remained unchanged (HS-fraction for α4(R336H)β2 [30:1] = 30 ± 5%; α4β2(V337G) [30:1] = 26 ± 6%; wild type α4β2 [30:1] = 14 ± 3%). This observation was not statistically significant (Figure 2C and Table 1), but the subtle increase in the amount HS-phase function suggested that the NFLE mutants might induce a shift in isoform expression. In later experiments, we directly measured the expression of the HS- and LS- isoforms via application of sazetidine-A.
Overall, these results demonstrated that, unlike TM-domain NFLE mutations previously studied, the α4(R336H) and β2(V337G) mutations did not alter ACh potencies in any of the three preparations. Instead, they may have increased the amount of HS-like phase function in the 30:1 [α4:β2] preparation.
3.2 C2 NFLE mutant subunits enhance ACh-induced peak currents
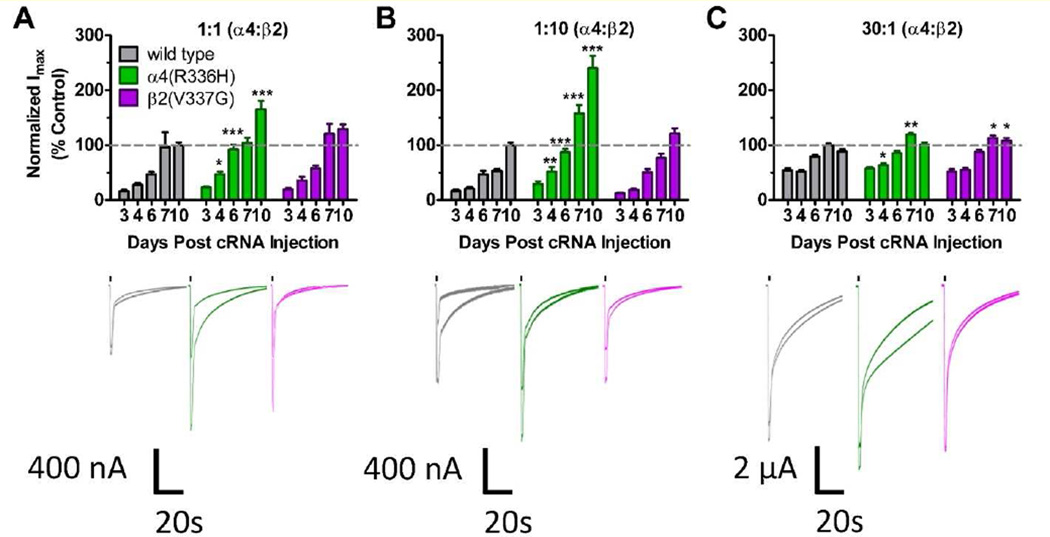
A second frequent effect of TM-domain NFLE mutations is to alter the magnitude of ACh-induced macroscopic currents compared to those produced by wild type α4β2-nAChR, as outlined in the Introduction. Accordingly, the maximum amount of function (Imax) that could be induced with ACh over a ten-day time course was measured using α4β2-nAChR expressed in Xenopus laevis oocytes (Figure 3 and Table 2). Responses were evaluated on 3, 4, 6, 7 and 10 days post cRNA injections, normalized to α4β2 wild type peak current responses on the day that oocytes had the most amount of function, and compared within and across wild type and C2 NFLE mutant groups. For wild type control α4β2-nAChR in each stoichiometric ratio group, as expected, the amount of peak function significantly increased during the course of the experiment (Figures 3A – C and Table 2). The maximum peak ACh-induced response was reached on day 10 post cRNA injections in the 1:1 and 1:10 [α4:β2] preparations, and on day 7 in the 30:1 preparation. Our findings also revealed that the amount of peak function significantly increased during the test period, within the groups hosting either α4(R336H) or β2(V337G) subunits (Figures 3A – C and Table 2).
Figure 3. (2 column width). C2 NFLE mutant subunits enhanced ACh-induced maximal currents in α4β2-nAChR subunit preparations.
Maximum peak ACh-induced function (Imax) was determined for wild type and mutant receptors expressed in unbiased (1:1 [α4:β2]) or biased (1:10 or 30:1 [α4:β2]) preparations in Xenopus oocytes. An increase in function from days 3 – 10 was seen across all groups, for all three preparations. A and B) The α4(R336H) mutation significantly enhanced ACh induced peak currents compared to wild type α4β2-nAChR, in both the 1:1 and 1:10 preparations. C) In the 30:1 preparation, both of the C2 NFLE mutations enhanced ACh peak currents compared to wild type α4β2-nAChR. Values are mean ± S.E.M and are quantified in Table 2. (N = 6 – 7, n = 36 – 49). Significant changes are increased Imax in C2 NFLE stoichiometries compared to nAChR expressed from wild type subunits in the corresponding preparation and are noted as follows: * P < 0.05; ** P < 0.01; *** P < 0.0001 (one-way ANOVA with Tukey’s post hoc test result values are reported in the Table 2 caption). Averaged traces below each graph show Imax responses for each construct at days 7 and 10 following cRNA injection (when differences between the wild-type α4β2-nAChR populations and those incorporating mutant subunits were most pronounced. The bars above each pair of traces depict 1s drug applications.
Table 2.
Effects of C2 NFLE mutant subunits on α4β2-nAChR ACh induced peak currents.
| Day 3 | Day 4 | Day 6 | Day 7 | Day 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subunits | Ratio (α4:β2) |
% Normalized Peak Function [Imax (µA)] |
N (n) |
% Normalized Peak Function [Imax (µA)] |
N (n) |
% Normalized Peak Function [Imax (µA)] |
N (n) |
% Normalized Peak Function [Imax (µA)] |
N (n) |
% Normalized Peak Function [Imax (µA)] |
N (n) |
| α4:β2 | 1:1 | 16 ± 3 [0.28 ± 0.08] |
7 (49) |
28 ± 3 [0.32 ± 0.07] |
7 (44) |
47 ± 4 [0.55 ± 0.11] |
7 (42) |
96 ± 27 [0.76 ± 0.13] |
7 (44) |
100 ± 5 [1.05 ± 0.14] |
7 (41) |
| α4(R336H)β2 | 1:1 | 23 ± 2 [0.31 ± 0.05] |
7 (44) |
47 ± 5* [0.55 ± 0.09] |
7 (43) |
92 ± 9*** [0.88 ± 0.10] |
7 (43) |
100 ± 10 [1.15 ± 0.15] |
7 (39) |
170 ± 15*** [1.77 ± 0.20] |
6 (36) |
| α4:β2(V337G) | 1:1 | 20 ± 3 [0.30 ± 0.07] |
7 (47) |
36 ± 6 [0.55 ± 0.15] |
6 (37) |
57 ± 5 [0.84 ± 0.14] |
6 (37) |
120 ± 18 [1.06 ± 0.14] |
7 (43) |
130 ± 9 [1.48 ± 0.25] |
6 (38) |
| α4:β2 | 1:10 | 16 ± 3 [0.14 ± 0.03] |
7 (41) |
21 ± 3 [0.22 ± 0.04] |
7 (42) |
47 ± 6 [0.55 ± 0.13] |
7 (43) |
53 ± 4 [0.51 ± 0.09] |
7 (43) |
100 ± 5 [1.08 ± 0.22] |
7 (40) |
| α4(R336H)β2 | 1:10 | 29 ± 5 [0.28 ± 0.08] |
7 (41) |
51 ± 9** [0.47 ± 0.14] |
7 (42) |
87 ± 6*** [0.72 ± 0.11] |
7 (44) |
160 ± 16*** [1.1 ± 0.17] |
7 (43) |
240 ± 23*** [1.59 ± 0.27] |
7 (48) |
| α4:β2(V337G) | 1:10 | 12 ± 2 [0.077 ± 0.010] |
7 (41) |
18 ± 2 [0.12 ± 0.02] |
7 (38) |
50 ± 6 [0.31 ± 0.05] |
7 (38) |
77 ± 8 [0.53 ± 0.07] |
7 (43) |
120 ± 9 [0.83 ± 0.10] |
7 (47) |
| α4:β2 | 30:1 | 54 ± 3 [3.6 ± 0.2] |
7 (42) |
52 ± 3 [3.5 ± 0.2] |
7 (42) |
79 ± 3 [5.2 ± 0.2] |
7 (43) |
100 ± 3 [6.7 ± 0.3] |
7 (42) |
88 ± 4 [5.9 ± 0.3] |
7 (42) |
| α4(R336H)β2 | 30:1 | 57 ± 3 [3.7 ± 0.2] |
7 (40) |
63 ± 3* [4.3± 0.3] |
7 (42) |
85 ± 5 [5.6 ± 0.3] |
7 (42) |
120 ± 2** [7.9 ± 0.2] |
7 (42) |
100 ± 5 [6.7 ± 0.3] |
7 (42) |
| α4:β2(V337G) | 30:1 | 51 ± 5 [3.2 ± 0.3] |
7 (36) |
55 ± 3 [3.7 ± 0.3] |
6 (42) |
87 ± 5 [5.7 ± 0.3] |
7 (43) |
110 ± 5* [7.2 ± 0.3] |
7 (40) |
110 ± 5* [7.0 ± 0.3] |
7 (45) |
Wild type and mutant subunits were expressed in Xenopus laevis oocytes using 1:1, 1:10 or 30:1 [α4:β2] cRNA injection ratios. A 1s application of a fully efficacious dose of ACh was used to determine the maximal ACh-induced peak current (Imax) on days 3, 4, 6, 7 and 10 post cRNA injections. Responses were then normalized to wild type values on the day that the oocytes had the greatest amount of function (Day 10 for 1:1 and 1:10, and Day 7 for 30:1 preparations). Initially, a two-way ANOVA was performed to determine the effect of the NFLE mutations and time post cRNA injections on Imax values. Both factors significantly altered the Imax values ([1:1 receptor subunit F2,86 = 8.70, P = 0.0004; time post injection F4 86 = 55.50, P < 0.0001; interaction receptor subunit x time post injection F8,86 = 1.79, P = 0.090]; [1:10 receptor subunit F2,90 = 79.35, P < 0.0001; time post injection F4,90 = 111.90, P < 0.0001; interaction receptor subunit x time post injection F8,90 = 10.24, P < 0.0001]; [30:1 receptor subunit F2,89 = 9.93, P = 0.0001; time post injection F4,89 = 125.4, P < 0.0001; interaction receptor subunit x time post injection F8,89 = 1.757, P = 0.0964]). Significant C2 NFLE mutant subunit driven enhancements of Imax values, compared to wild type function on the same day in the same injection preparation, were found (one-way ANOVA with Tukey’s post hos test: [1:1 Day 3 F2,18 = 1.68, P = 0.21; Day 4 F2,17 = 4.07, P = 0.036; Day 6 F2,17 = 13.23, P = 0.0003; Day 7 F2,18 = 0.40, P = 0.68; Day 10 F2,16 = 10.19, P = 0.0014]; [1:10 Day 3 F2,18 = 5.44, P = 0.014; Day 4 F2,18 = 11.09, P = 0.0007; Day 6 F2,18 = 12.65, P = 0.0004; Day 7 F2,18 = 28.33, P < 0.0001; Day 10 F2,18 = 27.02, P < 0.0001]; [30:1 Day 3 F2,18 = 0.90, P = 0.42; Day 4 F2,17 = 3.85, P = 0.042; Day 6 F2,18 = 1.10, P = 0.35; Day 7 F2,18 = 7.85, P = 0.0035; Day 10 F2,18 = 4.64, P = 0.024]. Raw Imax values are shown in italicized brackets beneath %Normalized Peak Function value.
All values are the mean ± S.E.M. One-way ANOVA with Tukey’s post hoc analysis significant effects are noted as follows:
P < 0.05;
P < 0.01;
P < 0.0001.
Uniquely, expression of the α4(R336H) mutation in the 1:1 [α4(R336H):β2] preparation resulted in a significant enhancement in Imax on days 4, 6 and 10 compared to wild type α4β2 nAChR (Figure 3A). In the 1:10 [α4(R336H):β2] preparation, the α4(R336H) mutation caused significant increases in Imax on days 4, 6, 7 and 10 compared to α4β2 receptors (Figure 3B). The α4(R336H) mutation when expressed using the 30:1 [α4(R336H):β2] expression ratio, caused a significant increase in Imax on days 4 and 7 when compared to wild type α4β2 receptors (Figure 3C).
In contrast, Imax responses appeared unaffected by expression of the β2(V337G) mutation in the 1:1 and 1:10 [α4:β2(V337G)] preparations. However, incorporation of β2(V337G) subunits into the 30:1 preparation did cause a significant increase in peak function on days 7 and 10 (Figure 3C and Table 2).
Overall, the incorporation of the α4(R336H) C2 NFLE mutant subunit consistently amplified ACh-induced functional responses across each of the stoichiometries tested, while β2(V337G)-driven Imax enhancements were specific for the 30:1 preparation.
3.3 Direct measurement of stoichiometric shifts induced by the C2 NFLE mutant subunits
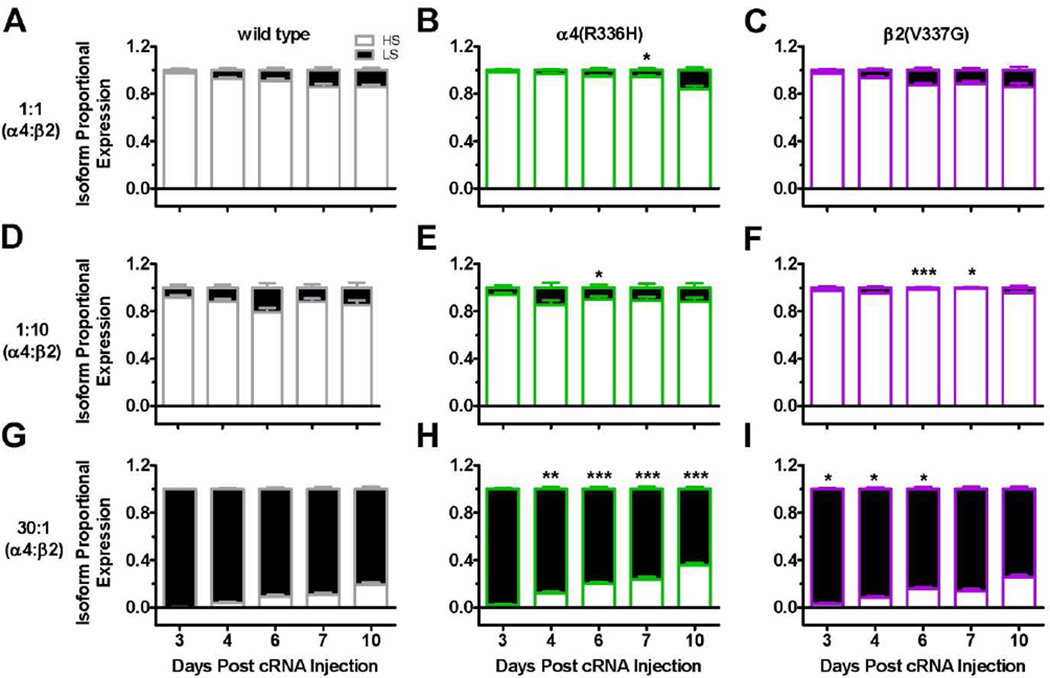
As shown in the preceding section, incorporation of either C2-loop NFLE mutant subunit significantly increased ACh Imax values compared to those measured from wild type α4β2-nAChR. We have previously shown that the concatenated LS-isoform α4β2-nAChR produce approximately five times more function per-receptor than their concatenated HS-isoform counterparts (Eaton et al., 2014). Using a loose subunit approach, one possible explanation of the observed C2-NFLE driven increase in macroscopic current could be a shift to expression of a greater proportion of LS (more functional) isoform α4β2-nAChR. This hypothesis was especially attractive given a previous publication indicating that TM-domain NFLE mutations favor expression of the LS, (α4)3(β2)2, isoform (Son et al., 2009). To measure any changes in isoform expression directly, the highly HS-selective agonist sazetidine-A was employed (see Methods section 2.4 for details). Comparisons were performed within each stoichiometric preparation and across the ten-day time course.
As indicated by our earlier results (see concentration-response curves in Figure 2), the HS-isoform was predominantly expressed in wild type 1:1 and 1:10 [α4:β2] preparations, although a slight increase in LS-isoform nAChR expression was seen at later time points (Figures 4A and D). Also as indicated by our initial concentration-response curves results, expression of the LS-isoform was prevalent in the 30:1 [α4:β2] wild type system. In the 30:1 [α4:β2] preparation, mirroring the effect in the HS-isoform-favoring preparations, the proportion of the alternative HS-isoform expression also increased over time (Figure 4G). In the 30:1 [α4:β2] preparation, the wild type HS-isoform was expressed minimally on day 3 (0.2 ± 0.1%) reaching a maximum of 19 ± 2% on day 10 (Figure 4G).
Figure 4. (2 column width). C2 NFLE mutant subunits preferentially enhanced HS-isoform α4β2*-nAChR expression.
Xenopus oocytes injected with wild type or mutant subunit cRNA in unbiased (1:1 [α4:β2]) or biased (1:10 or 30:1 [α4:β2]) preparations were exposed to a 1s application of a fully efficacious concentration of ACh, a 60s wash and then a further 1s sazetidine-A application. Sazetidine-A is an α4(+)/(−)β2-interface- (HS-phase function) selective agonist. The maximal peak current induced by sazetidine-A was measured, compared to the ACh Imax response and used to determine the percentage of the nAChR population represented by HS- [(α4)2(β2)3] versus LS- [(α4)3(β2)2] isoform nAChR (see Materials and Methods). A two-way ANOVA with Bonferroni post hoc test revealed significant effects of the NFLE mutants and time post cRNA injections ([1:1 injection preparation: receptor subunit: F2,90 = 5.25, P = 0.0070; time post cRNA injection: F4,90 = 20.03, P < 0.0001; interaction receptor subunit x time post cRNA injection: F8,90 = 1.60, P = 0.12]; [1:10 injection preparation: receptor subunit: F2,96 = 22.25, P < 0.0001; time post cRNA injection: F4,96 = 2.00, P = 0.10; interaction receptor subunit x time post cRNA injection: F8,96 = 1.44, P = 0.19]; [30:1 injection preparation: receptor subunit: F2,95 = 50.17, P < 0.0001; time post cRNA injection: F4,95 = 106.1, P < 0.0001; interaction receptor subunit x time post cRNA injection: F8,95 = 3.68, P = 0.0009]). In the 1:1 and 1:10 preparations, the HS-isoform was predominantly expressed during the entire 10-day time course (Figure 4A – F). Conversely, in the 30:1 preparation, the LS-isoform was the principal isoform expressed, however the HS-isoform expression increased with time (Figure 4G – I). A – C) In the 1:1 preparation, the α4(R336H) mutation significantly enhanced expression of HS-isoform α4β2*-nAChR (Day 7 post cRNA injection one-way ANOVA with Tukey’s post hoc test: F2,18 = 4.33, P = 0.029). D – F) Both C2 NFLE mutations enhanced the HS-isoform expression in the 1:10 preparation (Day 6 post cRNA injection one-way ANOVA with Tukey’s post hoc test: F2,21 = 14.35, P = 0.0001; Day 7 post cRNA injection one-way ANOVA with Tukey’s post hoc test: F2,18 = 6.29, P = 0.0085). G – I) In the 30:1 preparation, the C2 NFLE mutations significantly increased HS-isoform expression with the α4(R336H) mutation having the greatest effect (one-way ANOVA with Tukey’s post hoc test: Day 3 post cRNA injection: F2,18 = 4.99, P = 0.019; Day 4: F2,20 = 8.34, P = 0.0023; Day 6: F2,21 = 10.08, P = 0.0009; Day 7: F2,18 = 13.20, P = 0.0003; Day 10: F2,18 = 18.88, P < 0.0001). Significance in each case was determined by comparison of HS-isoform expression between C2 NFLE mutant and wild type α4β2-nAChR on the same day, and within the same injection-ratio preparations. Values are the mean ± S.E.M. (N = 6 – 7, n = 36 – 49). One-way ANOVA with Tukey’s post hoc analysis significant findings are noted as follows: * P < 0.05; ** P < 0.01; *** P < 0.0001.
Incorporation of C2 NFLE mutant subunits significantly increased the proportion of HS-isoform α4β2-nAChR in all tested stoichiometries when compared to wild type subunits, with the sole exception of the 1:1 [α4:β2(V337G)] preparation (Figure 4C). In the 1:1 and 1:10 [α4(R336H):β2] preparations, the α4(R336H) mutation significantly increased the expression of the HS-isoform on day 7 and 6 post cRNA injections compared to wild type nAChR (Figures 4B and 4E). The β2(V337G) mutation significantly enhanced the HS-isoform expression on days 6 and 7 post cRNA injection in the 1:10 [α4:β2(V337G)] preparation (Figure 4F).
In the 30:1 preparation, the α4(R336H) mutation caused significant enhancement of the HS-isoform expression on days 4 – 10 post cRNA injection (Figure 4H). The β2(V337G) mutation also increased the proportion of HS-isoform expression, reaching significance on days 3 – 6 post cRNA injection (Figure 4I). Thus, while the C2 NFLE mutants do indeed alter HS and LS expression ratios, they do so to favor the HS-isoform, the opposite direction to that shown for TM-domain mutants (Son et al., 2009).
3.4 The C2 NFLE mutant subunits alter partial agonist efficacy in the LS-favoring preparation
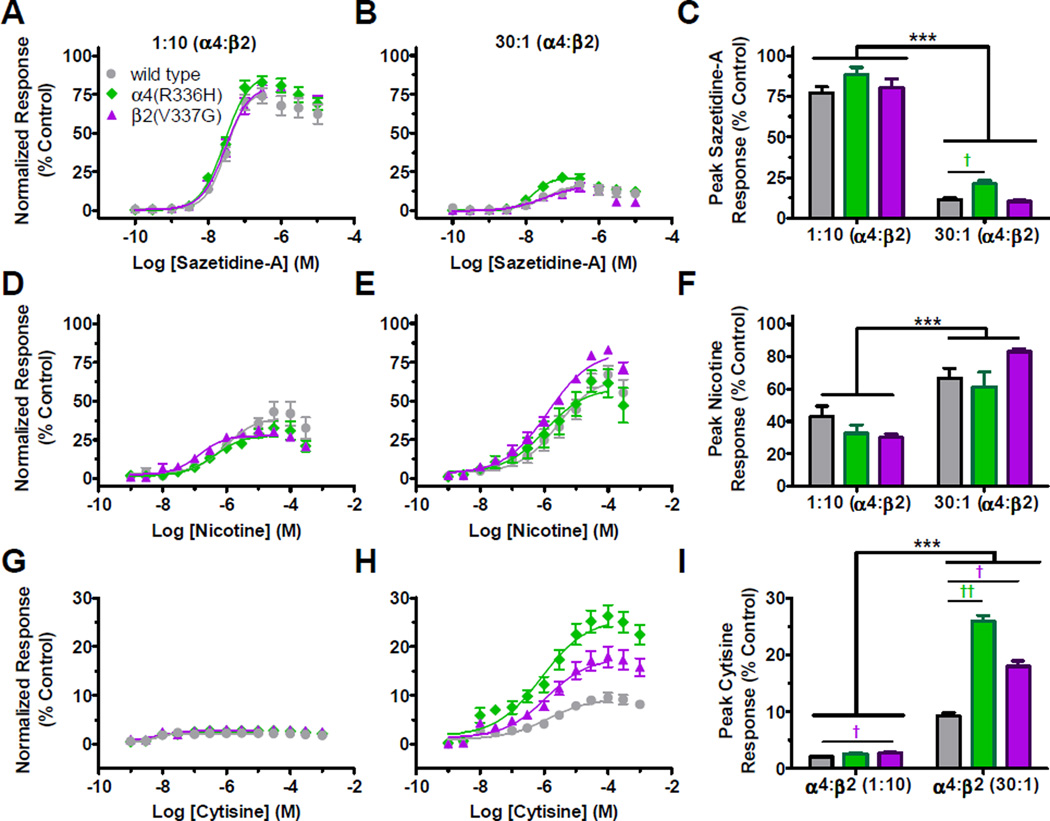
Nicotine has previously been shown to reduce seizure rates for carriers of NFLE mutations, and has been used as a self-medication treatment strategy (Brodtkorb and Picard, 2006; Willoughby et al., 2003). While prior investigations of the TM-domain NFLE mutations demonstrated changes in ligand potency (especially with regards to nicotine), this aspect of C2 NFLE mutant subunit effects has previously not been studied. The previous examinations of TM-domain NFLE mutation effects were performed in unbiased subunit-ratio expression systems (Hoda et al., 2008; Kuryatov et al., 1997); this complicates interpretation of potential differences caused by NFLE mutants in ligand potency and efficacy between the HS- and LS-isoforms. Accordingly, we evaluated the C2 NFLE mutations using the biased 1:10 and 30:1 preparations (Figure 5 and Table 3). The nAChR competitively-binding agonists chosen were sazetidine-A (which preferentially activates HS-isoform α4β2-nAChR), nicotine and cytisine, which preferentially activates LS-isoform α4β2-nAChR (Eaton et al., 2014; Moroni et al., 2006b; Zwart et al., 2008). In all cases, efficacy values were derived by comparison to ACh.
Figure 5. (2 column width). C2 NFLE mutant subunits do not affect agonist potency but do enhance efficacy of some partial agonists compared to ACh.
Wild type and mutant nAChR were expressed in biased (α4:β2 [1:10] or [30:1]) preparations in Xenopus oocytes. Receptor-expressing oocytes were exposed to a 1s application of increasing concentrations of several partial agonists. Agonists were tested on the same day (day 6) post nAChR subunit cRNA injection for each replicate experiment. Partial agonist responses were normalized to a fully efficacious concentration of ACh. A and B) C2 NFLE mutations had no effect on sazetidine-A pEC50 values. C) The α4(R336H) mutation enhanced sazetidine-A efficacy at 0.1 µM in the 30:1 preparation. D and E) No effect of the C2 NFLE mutations was seen on nicotine pEC50 values. F) Nicotine efficacy was enhanced for receptors expressed in the 30:1 preparation. G and H) No effect of the mutations was seen on cytisine potency. I) The C2 NFLE mutations increased cytisine efficacy in the 1:10 (β2(V337G)) and 30:1 (α4(R336H) and β2(V337G)) preparations. pEC50 and Hill slopes (nH) values are reported in Table 3, along with details of the statistical analysis. %Control is the Iligand response /Imax Ach. Values are the mean ± S.E.M. (N = 2, n = 5 – 8). Significant changes are noted as follows: *, † P < 0.05; **, †† P < 0.01; ***, ††† P < 0.0001. * Indicates significant effects between preparations (two-way ANOVA with Bonferroni post hoc test) and † indicates significance between groups within a preparation (one-way ANOVA with Tukey’s post hoc test).
Table 3.
NFLE mutant subunit effects on partial agonist potency, Hill slopes (nH) and efficacy.
| Sazetidine-A | Nicotine | Cytisine | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subunits | Ratio (α4:β2) |
pEC50 ± SEM |
nH ± SEM | Normalized Peak Response (% Control) |
pEC50 ± SEM |
nH ± SEM | Normalized Peak Response (% control) |
pEC50 ± SEM |
nH ± SEM | Normalized Peak Response (% control) |
|||||
| α4:β2 | 1:10 | 7.51 ± 0.05 | 1.57 ± 0.28 | 77 ± 4 |
|
6.04 ± 0.18 |
|
0.89 ± 0.31 | 40 ± 3 |
|
8.24 ± 0.13 |
|
~2 | 2.1 ± 0.1 |
|
| α4(R336H)β2 | 1:10 | 7.55 ± 0.06 | 1.32 ± 0.21 | 88 ± 4 | 6.36 ± 0.18 | 0.93 ± 0.32 | 28 ± 2 | 8.22 ± 0.26 | 1.35 ± 0.74 | 2.6 ± 0.1 | |||||
| α4:β2(V337G) | 1:10 | 7.53 ± 0.07 | 1.34 ± 0.26 | 81 ± 5 | 6.87 ± 0.14 | 1.07 ± 0.32 | 28 ± 1 | 8.20 ± 0.45 | 0.97 ± 0.58 | 2.8 ± 0.1† | |||||
| α4:β2 | 30:1 | 7.43 ± 0.19 | 1.08 ± 0.42 | 11 ± 1 | 5.58 ± 0.19 | 0.74 ± 0.23 | 65 ± 6 | 5.89 ± 0.18 | 0.64 ± 0.17 | 9.3 ± 0.5 | |||||
| α4(R336H)β2 | 30:1 | 7.79 ± 0.08 | 1.74 ± 0.46 | 21 ± 2† | 5.96 ± 0.29 | 0.69 ± 0.31 | 59 ± 7 | 6.08 ± 0.18 | 0.60 ± 0.15 | 26 ± 1†† | |||||
| α4:β2(V337G) | 30:1 | 7.50 ± 0.25 | 0.88 ± 0.37 | 10 ± 1 | 5.89 ± 0.08 | 0.64 ± 0.08 | 82 ± 3 | 5.93 ± 0.18 | 0.66 ± 0.18 | 18 ± 1† | |||||
Wild type and mutant nAChR subunits were expressed in Xenopus oocytes in a 1:10 or 30:1 [α4:β2] cRNA injection ratio. Experimental partial agonist concentration-response curve details can be found in the Materials and Methods and Figure 5 legend. All data were best fit using a monophasic logistic equation. Nicotine and cytisine pEC50 values for were significantly enhanced for receptors expressed in the 1:10 preparation (predominantly expressed the HS [(α4)2(β2)3] isoform), but sazetidine-A apparent potency was similar between the 1:10 and 30:1 preparations (two-way ANOVA with Bonferroni post hoc test: [nicotine: receptor subunit F2,6 = 4.71, P = 0.059; cRNA injection preparation F1,6 = 16.10, P = 0.0070; interaction receptor subunit x cRNA injection preparation F2,6 = 1.43, P = 0.31]; [cytisine: receptor subunit F2,6 = 0.12, P = 0.89; cRNA injection preparation F1,6 = 111.3, P < 0.0001; interaction receptor subunit x cRNA injection preparation F2,6 = 0.038, P = 0.96]; [sazetidine-A: receptor subunit F2,6 = 1.20, P = 0.37; cRNA injection preparation F1,6 = 0.15, P = 0.71; interaction receptor subunit x cRNA injection preparation F2,6 = 0.80, P = 0.49]). Within each cRNA injection ratio, the NFLE mutant subunits had no effect on partial agonist pEC50 values when compared to wild type values (one-way ANOVA with Tukey’s post hoc test: [sazetidine-A 1:10 preparation F2,3 = 0.11, P = 0.90; 30:1 preparation F2,3 = 1.10, P = 0.44]; [nicotine 1:10 preparation F2,3 = 6.33, P = 0.084; 30:1 preparation F2,3 = 0.97, P = 0.47]; [cytisine 1:10 preparation F2,3 = 0.0046, P = 1.00; 30:1 preparation F2,3 = 0.29, P = 0.77]). Partial agonist nH values were unaffected by the cRNA injection ratio or by the NFLE mutant subunits (two-way ANOVA with Bonferroni post hoc test: [sazetidine-A: receptor subunit F2,6 = 0.75, P = 0.51; cRNA injection preparation F1,6 = 0.39, P = 0.55; interaction receptor subunit x cRNA injection preparation F2,6 = 1.11, P = 0.39]; [nicotine: receptor subunit F2,6 = 0.018, P = 0.98; cRNA injection preparation F1,6 = 1.51, P = 0.27; interaction receptor subunit x cRNA injection preparation F2,6 = 0.13, P = 0.88]; [cytisine: receptor subunit F2,4 = 0.56, P = 0.61; cRNA injection preparation F1,4 = 3.98, P = 0.12; interaction receptor subunit x cRNA injection preparation F2,4 = 0.57, P = 0.61]). Sazetidine-A efficacy was significantly enhanced in the 1:10 preparation, demonstrating this ligands selectivity for the α4β2 HS- [(α4)2(β2)3] isoform, and there appeared to be an effect of the NFLE mutant subunits on sazetidine-A efficacy compared to wild type controls (two-way ANOVA with Bonferroni post hoc test: receptor subunit F2,6 = 6.83, P = 0.028; cRNA injection preparation F1,6 = 713.4, P < 0.0001; interaction receptor subunit x cRNA injection preparation F2,6 = 0.27, P = 0.77). One-way ANOVA with Tukey’s post hoc analysis confirmed that the α4(R336H) mutant subunit significantly enhanced sazetidine-A efficacy compared to wild type receptors, but only in the 30:1 preparation (one-way ANOVA with Tukey’s post hoc analysis: [1:10 injection ratio F2,3 = 1.845, P = 0.30]; [30:1 injection ratio F2,3 = 18.50, P = 0.021]). Nicotine efficacy was significantly enhanced in the 30:1 preparation compared to the 1:10 preparation (two-way ANOVA with Bonferroni post hoc test: receptor subunit F2,6 = 4.45, P = 0.065; cRNA injection preparation F1,6 = 124.7, P < 0.0001; interaction receptor subunit x cRNA injection preparation F2,6 = 7.31, P = 0.025). The NFLE mutant subunits caused no significant effects on nicotine efficacy within either cRNA injection ratio compared to wild type values (one-way ANOVA with Tukey’s post hoc analysis: [1:10 injection ratio F2,3 = 10.88, P = 0.042, but post hoc analysis could not distinguish a change in efficacy caused by the NFLE mutant subunits]; [30:1 injection ratio F2,3 = 5.18, P = 0.11]). The efficacy of cytisine was enhanced in the 30:1 injection ratio compared to the 1:10 preparation, and there was an effect of the NFLE mutant subunits preparation (two-way ANOVA with Bonferroni post hoc test: receptor subunit F2,6 = 100.1, P < 0.0001; cRNA injection preparation F1,6 = 929.7, P < 0.0001; interaction receptor subunit x cRNA injection preparation F2,6 = 89.62, P < 0.0001). The α4(R336H) mutation significantly enhanced cytisine efficacy, but only in the 30:1 preparation (one-way ANOVA with Tukey’s post hoc analysis: 30:1 cRNA preparation F2,3 = 93.02, P = 0.0019). The β2(V337G) mutant subunit enhanced cytisine efficacy in both the 1:10 and 30:1 preparations compared to wild type receptors (one-way ANOVA with Tukey’s post hoc analysis: [1:10 cRNA preparation F2,3 = 13.00, P = 0.033]; [30:1 cRNA preparation F2,3 = 96.36, P = 0.0019]).
All values are the mean ± S.E.M. Significant changes are noted as follows: *, † P < 0.05; **, †† P < 0.01; ***, ††† P < 0.0001. * Indicates significant effects between cRNA injection ratios (two-way ANOVA with Bonferroni post hoc analysis) and † indicates significance between groups within a preparation (one-way ANOVA with Tukey’s post hoc analysis).
In both the 1:10 and 30:1 [α4:β2] preparations, wild type α4β2 receptors produced similar sazetidine-A pEC50 values (7.5–7.4 [316–398 nM]) (Figures 5A, 5B and Table 3). C2 NFLE mutations had no effect on sazetidine-A potency. As anticipated, the efficacy of sazetidine-A was higher at the predominantly HS-isoform population (wild type [1:10] 77 ± 4% versus [30:1] 11 ± 1%) (Figure 5C and Table 3). Neither C2 NFLE mutation had an effect on sazetidine-A efficacy in the 1:10 [α4:β2] preparation (Figure 5C and Table 3). However, in the 30:1 [α4(R336H):β2] preparation, sazetidine-A efficacy was significantly higher (21 ± 2%) compared to wild type receptors (11 ± 1%) at 100 nM (Figure 5C and Table 3). As all sazetidine-A experiments were evaluated on day 6 post cRNA injection, this increase in α4(R336H) 30:1 efficacy could potentially be explained by the increased functional expression of the HS-isoform previously observed (see Figure 4H), since sazetidine-A efficacy at HS-isoform α4β2-nAChR is higher. However, the β2(V337G) mutant subunit did not significantly increase sazetidine-A efficacy despite it also increasing the expression of the HS-isoform (albeit to a lesser extent; see Figure 4I). These results suggest that the α4(R336H) increase in sazetidine-A efficacy could be due to enhanced HS-isoform expression, a change in the mutant receptor responsiveness to this ligand or a combination of both factors.
Nicotine has been shown previously to discriminate relatively poorly between HS- and LS- α4β2-nAChR isoforms (Marks et al., 1999). As expected, wild type nicotine potency and efficacy values were similar in both the 1:10 and 30:1 [α4:β2] preparations, although the (mostly LS) 30:1 wild type receptors did show a tendency towards increased efficacy compared to the predominantly HS 1:10 [α4:β2] population (students t-test: F1,1 = 1.21, P = 0.23) (Figure 5D – F and Table 3). Interestingly, a two-way ANOVA with Bonferroni post hoc test showed a significant difference in nicotine potency and efficacy between the HS 1:10 and LS 30:1 [α4:β2] injection ratios, but no C2 NFLE mutant subunit specific effect was found (Figure 5D – F and Table 3). However, a non-significant increase in nicotine efficacy in the 30:1 [α4:β2(V337G)] preparation compared to wild type α4β2-nAChR was observed (Figure 5F and Table 3). This possible β2(V337G) mutant subunit driven enhancement of LS-isoform nicotine efficacy cannot be due to mutant subunit-induced increase in the HS-isoform expression fraction (as measured in Figure 4 and considered in the Discussion), since nicotine efficacy is actually lower in the HS-isoform (Figure 5F).
Cytisine has previously been shown to have very low to no efficacy on HS receptors and ~22% efficacy on the LS-isoform (Moroni et al., 2006a). Here, we verified that our injection preparations with wild type subunit cRNA produced similar results, with cytisine having very low efficacy in the 1:10 [α4:β2] preparation and a higher efficacy in the wild type 30:1 [α4:β2] preparation (Figure 5I, Table 3). Cytisine was significantly more potent on receptors expressed in the 1:10 preparation compared to the 30:1 [α4:β2] injection ratio (Figure 5G, 5H and Table 3). Within each cRNA injection preparation, neither of the C2 NFLE mutant subunits had an effect on cytisine potency relative to that measured at the corresponding wild type α4β2-nAChR population (Figures 5G, 5H and Table 3). In the 1:10 [α4:β2(V337G)] expression method, the β2(V337G) mutation significantly increased cytisine efficacy compared to wild type receptors (Figure 5I and Table 3). Interestingly, both the α4(R336H) and the β2(V337G) expressed in the 30:1 preparation caused a significant enhancement in cytisine efficacy compared to wild type receptors. As for nicotine, the overall lower efficacy of cytisine at HS-isoform α4β2-nAChR (Figure 5I) shows that this observation can only be explained by a genuine C2 NFLE mutant subunit driven increase in cytisine efficacy relative to ACh at LS-isoform (α4)3(β2)2-nAChR.
Due to the cytoplasmic loop location of the α4(R336H) and β2(V337G) mutations investigated in this study, we expected to see no changes associated with ligands that bind in the extracellular portion of nAChR. Surprisingly, our findings show that the C2 NFLE mutations enhance partial agonist efficacy, particularly in the LS-favoring preparations, suggesting a change in mutant receptor responsiveness to particular ligands.
3.5 C2 NFLE mutant subunits affect DHβE antagonism in the LS-favoring preparation
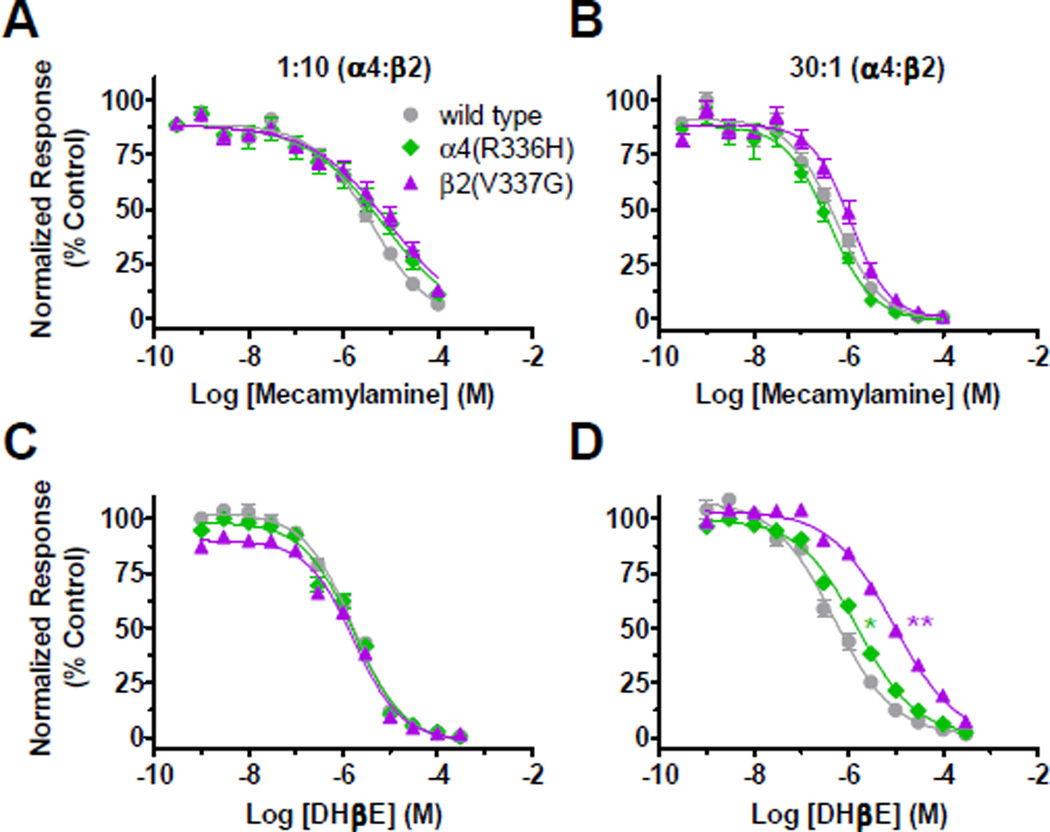
Effects of the non-competitive antagonist mecamylamine and the competitive antagonist dihydro-β-erythroidine (DHβE) were also tested using the 1:10 and 30:1 [α4:β2] subtype expression systems (Figure 6 and Table 4). The C2 NFLE mutations did not significantly affect mecamylamine pIC50 values in the 1:10 preparation, although a possible trend was seen towards decreased mecamylamine potency in the 30:1 [α4:β2(V337G)] preparations (Figures 6A, 6B and Table 4).
Figure 6. (1 column width). C2 NFLE mutant subunits affected DHβE antagonism of the α4β2-nAChR LS-isoform.
Xenopus oocytes were injected with wild type or mutant cRNA in biased (1:10 or 30:1 [α4:β2]) preparations. Each pharmacological ligand was tested on the same day post cRNA injection for each replicate experiment (mecamylamine day 6 and DHβE day 10). nAChR expressing oocytes were exposed to 1s co-applications of increasing concentrations of antagonist and the isoform-relevant EC90 ACh concentration (see Materials and Methods). A and B) C2 NFLE mutations had no effect on mecamylamine pIC50 values in either preparation. C and D) No effect was seen by expression of either C2 NFLE mutation on DHβE potency in the 1:10 preparation. However, both C2 NFLE mutation subunits significantly reduced DHβE pIC50 values in the 30:1 preparation. pIC50 and Hill slopes (nH) values are reported in Table 4, as are the details of the statistical analysis. Values are the mean ± S.E.M. (N = 2, n = 5 – 7). Significant changes are noted as follows: * P < 0.05; ** P < 0.01; *** P < 0.0001 (one-way ANOVA with Tukey’s post hoc test).
Table 4.
C2 NFLE mutant subunit effects on mecamylamine and DHβE antagonist pIC50 and Hill slope (nH) values.
| Mecamylamine | DHβE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subunits | Ratio (α4:β2) |
pIC50 ± SEM |
nH ± SEM | pIC50 ± SEM | nH ± SEM | ||||
| α4:β2 | 1:10 | 5.42 ± 0.10 |
|
0.71 ± 0.09 |
|
5.78 ± 0.04 |
|
0.83 ± 0.06 |
|
| α4(R336H)β2 | 1:10 | 5.19 ± 0.38 | 0.54 ± 0.15 | 5.77 ± 0.05 | 0.81 ± 0.07 | ||||
| α4:β2(V337G) | 1:10 | 5.06 ± 0.32 | 0.53 ± 0.12 | 5.77 ± 0.04 | 0.87 ± 0.07 | ||||
| α4:β2 | 30:1 | 6.27 ± 0.06 | 0.89 ± 0.06 | 6.31 ± 0.06 | 0.67 ± 0.06 | ||||
| α4(R336H)β2 | 30:1 | 6.41 ± 0.07 | 0.93 ± 0.13 | 5.81 ± 0.04† | 0.70 ± 0.04 | ||||
| α4:β2(V337G) | 30:1 | 5.96 ± 0.06 | 1.06 ± 0.14 | 5.05 ± 0.07†† | 0.65 ± 0.05 | ||||
Biased (1:10 or 30:1 [α4:β2]) expression of wild type and C2 NFLE mutant subunits was performed in Xenopus oocytes. Antagonists were co-applied with an ACh EC90 concentration and data were best fit using a monophasic logistic equation. Further details regarding the concentration-response profiles can be found in the Materials and Methods and Figure 6 legend. Mecamylamine pIC50 values were significantly different between the 1:10 and 30:1 expression preparations (two-way ANOVA with Bonferroni post hoc test: receptor subunit F2,6 = 1.60, P = 0.28; cRNA injection preparation F1,6 = 33.65, P = 0.0012; interaction receptor subunit x cRNA injection preparation F2,6 = 0.50, P = 0.63). C2 NFLE mutations had no effect on mecamylamine pIC50 values in either preparation compared to wild type subunit containing receptors (one way ANOVA with Tukey post hoc test: [1:10 preparation: F2,3 = 0.44, P = 0.68]; [30:1 preparation F2,3 = 13.17, P = 0.033, with a Tukey’s test showing a significant effect of the mutant subunits being different from each other but not different from wild type receptors]). Mecamylamine nH values were significantly different between the cRNA injection preparations but no specific effect of the C2 NFLE mutant subunits was seen (two-way ANOVA with Bonferroni post hoc test: receptor subunit F2,6 = 0.18, P = 0.84; cRNA injection preparation F1,6 = 14.22, P = 0.0093; interaction receptor subunit x cRNA injection preparation F2,6 = 1.09, P = 0.39). No significant difference was detected between DHβE pIC50 values between isoform expression preparations but significant differences were observed between the C2 NFLE mutant subunits (two-way ANOVA with Bonferroni post hoc test: receptor subunit F2,6 = 77.42, P < 0.0001; cRNA injection preparation F1,6 = 1.54, P = 0.26; interaction receptor subunit x cRNA injection preparation F2,6 = 75.86, P < 0.0001). Both C2 NFLE mutations significantly reduced DHβE pIC50 values in the 30:1 preparation (one-way ANOVA with Tukey post hoc test: [1:10 preparation: F2,3 = 0.0083, P = 0.99]; [30:1 preparation F2,3 = 122.7, P = 0.0013]). DHβE nH values in the 1:10 preparation were significantly larger than the 30:1 preparation, but no effect of the C2 NFLE mutant subunits was detected (two-way ANOVA with Bonferroni post hoc test: receptor subunit F2,6 = 0.014, P = 0.99; cRNA injection preparation F1,6 = 11.38, P = 0.015; interaction receptor subunit x cRNA injection preparation F2,6 = 0.43, P = 0.67).
Significant changes are noted as follows: *, † P < 0.05; **, †† P < 0.01; ***, ††† P < 0.0001. * Indicates significant effects between preparations (two-way ANOVA with Bonferroni post hoc test) and † indicates significance between groups within a preparation (one-way ANOVA with Tukey’s post hoc test).
C2 NFLE mutations had no effect on DHβE potency using the 1:10 [α4:β2] expression system (Figure 6C and Table 4). However, in the 30:1 preparation, both C2 NFLE mutants significantly increased DHβE pIC50 values compared to wild-type α4β2-nAChR (Figure 6D and Table 4). This is another example of a C2 NFLE mutant subunit effect on nAChR responsiveness to a competitively-binding ligand.
3.6 Effects of the C2 NFLE mutant subunits on receptor cell-surface expression and per-receptor function
The overall increases in Imax (see Figure 3) caused by the C2 NFLE mutations could be induced by enhanced receptor surface expression, increased amount of function per-receptor or a combination of both effects. To address the possibility that the mutations altered nAChR surface expression, we measured cell-surface nAChR expression using a radiolabeled antibody, [125I]mAb 295, which is specific for correctly-folded β2 nAChR subunits (see Methods and Materials). To allow us to compare directly mutant subunit-induced changes in cell-surface receptor expression with functional changes, ACh Imax currents were measured in the same oocytes. Comparisons were made at 4, 6 and 10 days post cRNA injection, for each of the three receptor expression preparations (1:1, 1:10 and 30:1 [α4:β2]; Figure 7 and Table 5).
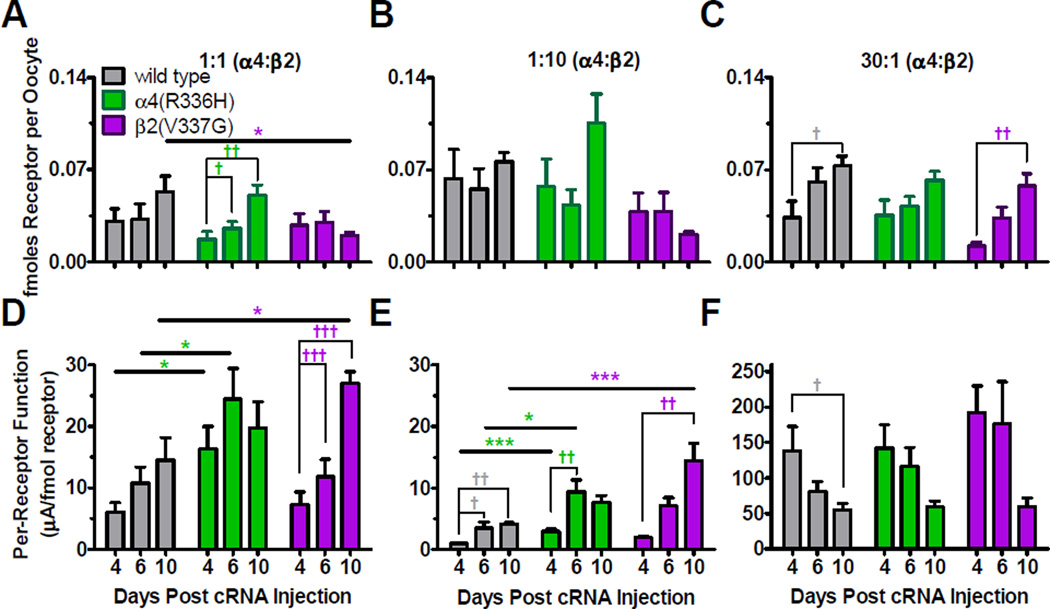
Figure 7. (2 column width). The β2(V337G) NFLE mutant subunit decreased α4β2-nAChR cell-surface expression and both C2 NFLE mutant subunits enhanced per-receptor function.
Xenopus oocytes were injected with wild type or mutant cRNA in unbiased (1:1 [α4:β2]) or biased (1:10 or 30:1 [α4:β2]) preparations. Peak ACh-induced function and the proportions of HS-to-LS-isoform expression ratios were measured as described in the legend to Figure 4, on days 4, 6 and 10 post cRNA injection. Using the same oocytes, cell-surface receptors were measured using [125I]mAb 295, a β2 selective antibody. Note that data were corrected for number of [125I]mAb 295 molecules bound to each isoform (three per (α4)2(β2)3 HS-isoform, and two for the (α4)3(β2)2 LS-isoform), and for the proportional expression of HS- and LS- isoform α4β2-nAChR. A – C) The amount of wild type and α4(R336H) containing receptors expressed on the surface of the oocyte tended to increase similarly with increasing days post cRNA injection. In the 1:1 preparation, expression of the β2(V337G) mutation caused a significant reduction in cell-surface receptors. D – E) Imax values were normalized to the amount of nAChR cell-surface expression for each construct. Significant increases in the amount of per-receptor function were noted in the HS-isoform-favoring preparations for both C2 NFLE mutations. F) The amount of per-receptor function decreased with time in the 30:1 preparation, likely due to the increased expression of the less functional HS-isoform with time. Specific [125I]mAb 295 binding per oocyte and Imax (nA) values are reported in Table 5, as are details of the statistical analysis applied. Values are the mean ± S.E.M. (N = 5 – 7, n = 30 – 43). * Indicates significant effects caused by the NFLE mutant subunits compared to wild type receptors on a specific day post cRNA injections (one-way ANOVA with Tukey’s post hoc test) and, † indicates significant differences due to the number of days post cRNA injections within a given receptor preparation (one-way ANOVA with Tukey’s post hoc test). Significant changes are noted as follows: *, †P < 0.05; **, †† P < 0.01; ***, ††† P < 0.0001.
Table 5.
Effects of C2 NFLE mutant subunits on function, cell-surface expression and per-receptor function.
| Day 4 | Day 6 | Day 10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subunits | Ratio (α4:β2) |
Specific [125I]mAb295 Binding (fmoles receptor/ oocyte) |
Per-Receptor Function (µA/fmoles receptor) |
N | Specific [125I]mAb295 Binding (fmoles receptor/oocyte) |
Per-Receptor Function (µA/fmoles receptor) |
N | Specific [125I]mAb295 Binding (fmoles receptor/ oocyte) |
Per-Receptor Function (µA/fmoles receptor) |
N |
| α4:β2 | 1:1 | 0.031 ± 0.010 | 6.1 ± 1.6 | 5 | 0.032 ± 0.012 | 11 ± 3 | 6 | 0.053 ± 0.012 | 14 ± 4 | 7 |
| α4(R336H)β2 | 1:1 | 0.017 ± 0.006 | 16 ± 4* | 5 | 0.025 ± 0.005 | 24 ± 5* | 6 | 0.050 ± 0.008†† | 23 ± 4 | 6 |
| α4:β2(V337G) | 1:1 | 0.028 ± 0.009 | 7.3 ± 2.0 | 5 | 0.030 ± 0.008 | 12 ± 3 | 6 | 0.020 ± 0.002* | 27 ± 2*,††† | 6 |
| α4:β2 | 1:10 | 0.063 ± 0.022 | 1.0 ± 0.1 | 5 | 0.055 ± 0.015 | 3.5 ± 0.9† | 6 | 0.076 ± 0.007 | 4.1 ± 0.2†† | 7 |
| α4(R336H)β2 | 1:10 | 0.058 ± 0.020 | 3.0 ± 0.4*** | 6 | 0.043 ± 0.012 | 9.4 ± 1.9*,†† | 6 | 0.105 ± 0.022 | 7.7 ± 1.1 | 6 |
| α4:β2(V337G) | 1:10 | 0.038 ± 0.014 | 2.0 ± 0.2 | 5 | 0.039 ± 0.014 | 7.1 ± 1.4 | 6 | 0.021 ± 0.002 | 15 ± 2.8**,†† | 7 |
| α4:β2 | 30:1 | 0.034 ± 0.012 | 138 ± 34 | 6 | 0.061 ± 0.010 | 81 ± 14 | 6 | 0.073 ± 0.007† | 55 ± 9† | 7 |
| α4(R336H)β2 | 30:1 | 0.036 ± 0.011 | 140 ± 30 | 6 | 0.043 ± 0.007 | 117 ± 26 | 6 | 0.062 ± 0.007 | 60 ± 8 | 6 |
| α4:β2(V337G) | 30:1 | 0.012 ± 0.003 | 192 ± 38 | 6 | 0.033 ± 0.008 | 176 ± 60 | 6 | 0.058 ± 0.009†† | 59 ± 12 | 6 |
Wild type and mutant constructs were expressed in Xenopus oocytes in a 1:1, 1:10 or 30:1 [α4:β2] cRNA injection ratio. Imax values and HS (α4)2(β2)3 versus LS (α4)3(β2)2 isoform ratios were determined as described in the legend to Figure 4. The same oocytes were then used to measure nAChR surface expression using [125I]mAb 295. Specific binding data was corrected for number of [125I]mAb 295 molecules bound to each isoform (three per (α4)2(β2)3 HS-isoform and two for the (α4)3(β2)2 LS-isoform), and the proportion of the HS- and LS- isoforms expressed within each group at each time point (described in legend to Figure 7, and in the Materials and Methods section). In the 1:10 and 30:1 preparations, the C2 NFLE mutant subunits and time post cRNA injections caused significant differences in cell-surface receptor expression (two-way ANOVA Bonferroni with Tukey’s post hoc test: [1:10 cRNA injection preparations: receptor subunit F2,45 = 4.26, P = 0.020; time post injection F2,45 = 1.363, P = 0.27; interaction receptor subunit x time post cRNA injection F4,45 = 1.67, P = 0.17]; [30:1 cRNA injection preparations: receptor subunit F2,44 = 4.37, P = 0.019; time post injection F2,44 = 12.76, P < 0.0001; interaction receptor subunit x time post cRNA injection F4,44 = 0.51, P = 0.73]. Results show that the α4(R336H) mutation had no significant effect on receptor cell-surface expression compared to wild type α4β2-nAChR at the same time-point and using the same subunit injection ratios. However, the β2(V337G) mutation significantly decreased receptor cell-surface expression relative to α4β2-nAChR wild type preparations, most-strikingly on day 10 post cRNA injections in the 1:1 preparation (one-way ANOVA: 1:1 preparation Day 10 post injection: F2,16 = 4.25, P = 0.033). The 1:1 α4(R336H):β2 preparation and wild type and α4β2(V337G) 30:1 receptors had significant increases in cell-surface protein expression on day 10 post cRNA injection (one-way ANOVA: [1:1 α4(R336H):β2 preparation days post injection: F2,14 = 7.03, P = 0.0077]; [30:1 α4:β2 preparation days post injection: F2,15 = 3.90, P = 0.043]; [30:1 α4:β2(V337G) preparation days post injection: F2,14 = 8.37, P = 0.0041]). Expression of the C2 NFLE mutant subunits and time post cRNA injection cause significant effects on the per-receptor function in all expression preparations (two-way ANOVA Bonferroni with Tukey’s post hoc test: [1:1 cRNA injection preparations: receptor subunit F2,43 = 8.00, P = 0.0011; time post injection F2,43 = 9.005, P = 0.0005; interaction receptor subunit x time post cRNA injection F4,44 = 2.58, P = 0.050]; [1:10 cRNA injection preparations: receptor subunit F2,45 = 9.24, P = 0.0004; time post injection F2,45 = 16.28, P < 0.0001; interaction receptor subunit x time post cRNA injection F4,45 = 4.18, P = 0.0058]; [30:1 cRNA injection preparations: receptor subunit F2,46 = 2.30, P = 0.1117; time post injection F2,46 = 8.66, P = 0.0006; interaction receptor subunit x time post cRNA injection F4,46 = 0.63, P = 0.64]. The α4(R336H) mutant subunit significantly enhanced the per-receptor function on days 4 and 6 post cRNA injection in both the 1:1 and 1:10 preparations (one-way ANOVA: [1:1 α4(R336H):β2 preparation Day 4 post injection: F2,12 = 4.64, P = 0.032]; [1:1 α4(R336H):β2 preparation Day 6 post injection: F2,15 = 4.38, P = 0.032]; [1:10 α4(R336H):β2 preparation Day 4 post injection: F2,13 = 11.48, P = 0.0013]; [1:10 α4(R336H):β2 preparation Day 6 post injection: F2,15 = 4.03, P = 0.040]). The β2(V337G) mutant subunit significantly enhanced the per-receptor function on day 10 post injection in the HS-favoring preparations (one-way ANOVA: [1:1 α4:β2(V337G) preparation Day 10 post injection: F2,16 = 3.98, P = 0.040]; [1:10 α4:β2(V337G) preparation Day 10 post injection: F2,17 = 8.76, P = 0.0024]). Per-receptor function significantly increased with days post cRNA injection in the 1:1 α4:β2(V337G), 1:10 α4:β2, 1:10 α4(R336H):β2, 1:10 α4:β2(V337G) and 30:1 α4:β2 preparations (one-way ANOVA: [1:1 α4:β2(V337G) preparation days post injection: F2,14 = 19.71, P < 0.0001]; [1:10 α4:β2 preparation days post injection: F2,15 = 8.55, P = 0.0033]; [1:10 α4(R336H):β2 preparation days post injection: F2,15 = 6.38, P = 0.0099]; [1:10 α4:β2(V337G) preparation days post injection: F2,15 = 9.11, P = 0.0026]; [30:1 α4:β2 preparation days post injection: F2,16 = 4.20, P = 0.034]).
All values are the mean ± S.E.M (N = 5 – 7). * Indicates significant effects caused by the NFLE mutant subunits compared to wild type receptors on a specific day post cRNA injections (one-way ANOVA with Tukey’s post hoc test), and † indicates significant differences due to the number of days post cRNA injections within a given receptor population (one-way ANOVA with Tukey’s post hoc test). Significant changes are noted as follows: *, †P < 0.05; **, †† P < 0.01; ***, ††† P < 0.0001.
Wild type cell-surface binding values for the 1:10 and 30:1 [α4:β2] preparations were similar to previous studies measuring surface binding of concatenated α4β2 HS and LS receptors (Figures 7A – C and Table 4) (Eaton et al., 2014). Wild type binding values did not change significantly between days 4, 6 and 10 in either the 1:1 or 1:10 [α4:β2] preparations (Figure 7A, 7B and Table 4). In the wild type 30:1 [α4:β2] preparation, the [125I]mAb 295 binding was significantly increased on day 10 versus day 4 post cRNA injection (Figure 7C and Table 4).
Substitution of the α4(R336H) mutant subunit had no effect on cell-surface binding across the time course in any of the preparations when compared to wild type receptors (Figures 7A – C and Table 4). In contrast, in the 1:1 and 1:10 preparations, the β2(V337G) mutation tended to diminish cell-surface binding during the ten day time course when compared to wild type receptor binding values measured on the same day (Figures 7A, 7B and Table 4). A statistically significant decrease in the β2(V337G) cell-surface binding was observed on day 10 in the 1:1 [α4:β2(V337G)] preparation when compared to the wild type 1:1 [α4:β2] group on the same day (Figures 7A and Table 4). In the 30:1 preparation, expression of either mutant subunit had no significant effect on cell-surface expression compared to wild type receptors (Figure 7C and Table 4). These results suggest that the α4(R336H) mutation does not modify receptor expression levels compared to wild type α4β2 receptors, while the β2(V337G) mutation decreases HS-isoform cell-surface expression in some cases.
The amount of function per unit of receptor was calculated to determine if changes in Imax (measured in Figure 3) were caused by alterations in receptor surface expression or per-receptor function, defined as µA (Imax) per fmoles receptor of specific binding (Figure 7D – F and Table 5). Note that surface receptor expression was calculated taking into account the proportions of HS versus LS α4β2-nAChR expression at each sampled time point (see Figure 4), and the fact that these isoforms provide 3 versus 2 [125I]mAb295 binding sites, respectively. The amount of per-receptor function increased with days post cRNA injection in all groups within the 1:1 and 1:10 [α4:β2] preparations, while a decrease was observed in the 30:1 preparation. In wild type 1:1 and 1:10 [α4:β2] preparations, the amount of per-receptor function tended to increase from days 4 to 10 post cRNA injection, reaching significantly higher levels in the 1:10 preparation on days 6 and 10 (Figures 7D, 7E and Table 5). The observed increase in perreceptor function observed in the wild type HS-isoform favoring (1:10 and 1:1 [α4:β2]) preparations could have been caused by increased expression of the more functional LS-isoform, as observed in Figure 4A and 4D. Wild type receptors expressed in the 30:1 [α4:β2] preparation had significantly decreased per-receptor function on day 10 compared to day 4 post cRNA injection (Figure 7F and Table 5), despite the enhancement of receptor cell-surface expression (Figure 7C). This is likely caused by increased expression of the less functional HS-isoform, as shown in Figure 4G.
In the 1:1 and 1:10 [α4(R336H):β2] preparations, the α4(R336H) mutation caused a significant increase in the per-receptor function on days 4 and 6 post cRNA injection when compared to wild type receptors on days 4 and 6 (Figure 7D, 7E and Table 5). Expression of the β2(V337G) mutation in the 1:1 and 1:10 preparations [α4:β2(V337G)] significantly enhanced the per-receptor function on day 10 compared to wild type receptors (Figures 7D, 7E and Table 5). No significant difference in per-receptor function was observed for either mutant in the 30:1 preparation when compared to wild type receptors (Figure 7D). Overall, our findings indicate that the amount of function per unit of receptor is enhanced by the C2 NFLE mutations, especially in the case of the HS-isoform α4β2-nAChR function associated with the (α4)2(β2)3 stoichiometry.
4. Discussion
4.1 Study scope and purpose
Our findings provide the first confirmation, and detailed characterization, of functional effects of a pair of NFLE-associated mutations (α4(R336H) and β2(V337G)) located in the large intracellular cytoplasmic loop (C2) domain of the nAChR α4 and β2 subunits. Effects on macroscopic functional parameters were compared to previous findings for ADNFLE-linked mutations found in α4 and β2 subunit transmembrane (TM) domains. Extending past the majority of previously-published studies on TM-domain ADNFLE-linked nAChR subunit mutants, effects on surface expression and the balance between HS- and LS- α4β2-nAChR isoforms were also determined, as were differential effects between the isoforms. This study demonstrates that changes to cytoplasmic loop residues can significantly alter α4β2-nAChR cell surface expression, isoform assembly and function per receptor. Comparisons to functional effects caused by TM-domain ADNFLE-linked nAChR subunit mutants indicate both differences and similarities in outcomes. It is likely that the points of similarity are particularly pertinent to AD/NFLE etiology.
4.2 Macroscopic function effects of C2 NFLE-linked nAChR subunit mutations
The macroscopic function outcomes of including either C2-domain mutant subunit closely resembled each other. Neither incorporation of the α4(R336H) nor the β2(V337G) subunit had any significant effect on agonist EC50 values for ACh at either HS- or LS- isoform α4β2-nAChR. This contrasts strongly with outcomes from previous investigations of TM-domain ADNFLE mutations, which typically report increased agonist potency. In particular, TM-domain-β2 mutant subunits consistently enhanced ACh potency and macroscopic ACh-induced currents (Bertrand et al., 2005; Bertrand et al., 2002; Hoda et al., 2008; Rodrigues-Pinguet et al., 2003; Steinlein et al., 2012a). Enhanced ACh sensitivity is also a common feature of TM-domain-α4 mutant subunit incorporation, although contradictory findings have been reported for α4(S248F) (Bertrand et al., 2002; Figl et al., 1998; Kuryatov et al., 1997; Rodrigues-Pinguet et al., 2003). Unlike for TM-domain-β2 subunits, TM-domain-α4 incorporation typically decreases or leaves unchanged macroscopic ACh-induced currents (Bertrand et al., 2002; Figl et al., 1998; Kuryatov et al., 1997; Rodrigues-Pinguet et al., 2003; Steinlein et al., 2012a; Steinlein et al., 1997; Weiland et al., 1996).
Effects of NFLE-associated mutant subunits on HS- and LS- α4β2-isoform expression and cell-surface expression are less-investigated. TM-domain-α4 subunits S247F, S252F, S256L and +L264 have been tested, with no effect reported on total and/or surface receptor expression (Figl et al., 1998; Kuryatov et al., 1997; Rodrigues-Pinguet et al., 2003). Intriguingly, three α4- and two β2- ADNFLE-mutant subunits have been shown to promote preferential intracellular assembly of LS-isoform α4β2-nAChR (Son et al., 2009). However, most previous studies used 1:1 [α4:β2] expression ratios (resulting in decreased control of isoform expression ratios), and did not distinguish if apparent shifts in EC50 values were due to changes in ligand potency per se, or altered isoform expression ratios. It is important to note that, in hindsight, several of the ACh concentration-response profiles within these previous studies appear biphasic, containing both HS- and LS- phase components, and that expression of some TM-domain ADNFLE mutations seemed to increase the HS-phase responses relative to controls (Bertrand et al., 2002; Hoda et al., 2008; Steinlein et al., 2012a). Further investigation may therefore be warranted to determine the effects of TM-domain ADNFLE-associated α4 and β2 subunit variants on HS- versus LS- isoform expression and, especially, functional ratios which may underpin some previous reports of TM-domain ADNFLE-associated mutant subunits producing enhanced ACh potency at α4β2-nAChR populations.
Incorporation of either C2-domain NFLE-linked subunit enhanced ACh-induced α4β2-nAChR macroscopic currents. This effect is more similar to those reported for TM-domain ADNFLE-linked mutations where, especially for β2 TM-domain mutant subunits as noted previously, similar effects have repeatedly been reported. It seems likely, therefore, that enhanced overall α4β2-nAChR function is a common contributor to AD/NFLE causation across the two classes (C2- and TM- domain) of AD/NFLE-linked nAChR subunit mutations.
4.3 α4β2-nAChR surface and isoform expression effects of C2-NFLE-linked nAChR subunit mutations
The overall effects on cell surface expression of the two C2-domain mutant subunits were very subtle. α4(R336H) subunit incorporation had no significant effect on surface expression compared to wild type α4β2-nAChR, while the β2(V337G) mutation slightly decreased α4β2-nAChR expression at the cell surface in the HS-biased preparation (Figure 7). These outcomes are broadly compatible with findings from earlier studies of TM-domain ADNFLE-associated mutations which showed no effect on total α4β2*-nAChR surface expression (Figl et al., 1998; Kuryatov et al., 1997; Rodrigues-Pinguet et al., 2003).
Effects were seen on the relative surface expression levels of HS- versus LS- isoform α4β2-nAChR. Both the α4(R336H) and β2(V337G) C2-domain mutants consistently favored expression of a higher proportion of HS-isoform α4β2-nAChR, regardless of the subunit injection ratios used (hinted at in Figure 2C, and directly measured in Figure 4). Interestingly, augmentation of HS-isoform expression was more pronounced in the preparation where the mutant subunit was injected in a greater quantity than the wild type subunit (e.g. β2(V337G) had more effect in the HS-favoring 1:10 injection ratio that predominantly produces the (α4)2(β2)3 stoichiometry, while α4(R336H) was more effective in oocytes predominantly expressing (α4)3(β2)2 nAChR). This likely indicates a gene-dose effect in both cases. Our findings are consistent with a recent study investigating the functional effects of the rare α4(R336C) mutation found to be underrepresented among dependent smokers (McClure-Begley et al., 2013). This study showed that the α4(R336C) mutation (located at the same amino acid position as the C2 NFLE α4(R336H) mutation) also enhanced the assembly of the HS-isoform (McClure-Begley et al., 2013). However, these effects are the opposite of the outcomes reported in a recent Förster resonance energy transfer study (Son et al., 2009), where multiple TM-domain ADNFLE mutations [α4(S247F), α4(S252L), α4(776ins3), β2(V287L) and β2(V287M)] shifted the stoichiometry expression ratio to favor the LS- [(α4)3(β2)2] isoform. It is important to note that the FRET technique used in Son et al. (2009) likely captures data from both intracellular and extracellular α4β2-nAChR populations, so it is possible that direct comparison to the present study’s surface-population expression data could be misleading. Nevertheless, disruption of the expressed HS- and LS- isoform ratio is a common feature across multiple AD/NFLE C2- and TM- domain mutant nAChR subunits. This may suggest that, regardless of direction, alteration of the HS:LS-isoform ratio may underlie the pathophysiology of AD/NFLE.
4.4 C2-domain mutations alter pharmacological parameters of competitive agonists and the competitive antagonist DHβE
Also similar between the α4(R336H) and β2(V337G) mutant subunits were changes in competitive partial-agonist and antagonist effects, primarily seen in the LS-isoform α4β2-nAChR population favored under the 30:1 expression ratio. Pharmacological evaluation revealed that the C2 NFLE mutants increased the relative efficacy of three partial agonists (sazetidine-A, nicotine and cytisine) relative to ACh at LS-isoform α4β2-nAChR (Figure 5). We observed increases in both sazetidine-A and cytisine efficacy with the C2 NFLE mutant subunits in the 30:1 preparation. In the 30:1 preparation, the α4(R336H) mutant subunit increased sazetidine-A efficacy, potentially caused by the enhanced expression of the more sazetidine-A efficacious HS-isoform (measured in Figure 4). However, the β2(V337G) mutant subunit also caused a significant increased expression of the HS-isoform (Figure 4I), but did not significantly alter sazetidine-A potency in the 30:1 [α4:β2(V337G)] preparation (Figure 5I). These results suggest that the increase in sazetidine-A α4(R336H) 30:1 efficacy could be due to either a change in expression of the HS-isoform or the functional outcome of agonist stimulation. Consistent with the idea that the C2 NFLE mutant subunit may alter agonist-induced function, both of the α4(R336H) and β2(V337G) mutant subunits expressed in the 30:1 preparation increased cytisine efficacy. This effect could not be due to the observed increase in HS-isoform expression (Figure 4), since cytisine is less efficacious on the HS-isoform. These findings suggest that the observed increase in nicotine and cytisine relative efficacy can only be due to a genuine receptor-level effect on competitive partial agonist efficacy. This observation was reinforced by the fact that the competitive antagonist DHβE potency was reduced in LS-isoform α4β2-nAChR hosting the two C2-domain mutants, but that of the non-competitive antagonist mecamylamine was not affected.
4.5 C2-domain mutations can significantly modify nAChR function and expression
The C2 loop is consistently the longest and most variable intracellular loop across the family of vertebrate nAChR subunits (Stokes et al., 2015). Despite this variability, the regions of the loop closest to the bordering TM3 and TM4 helices exhibit considerable sequence conservation. The better studied of these conserved regions is a highly-conserved membrane-associated α-helix (MA) close to the TM4 helix that is thought to form part of an intracellular portal through which ion flux occurs (Unwin, 2005), and which has been shown to play an important role in controlling ACh-induced peak membrane currents, unitary conductance and protein interactions (Hales et al., 2006; Pollock et al., 2009). The C2 NFLE mutations are located in the second highly conserved part of the cytoplasmic loop (Kuo et al., 2005; Stokes et al., 2015), close to the TM3 helix (Figure 1C). Given this location, it is tempting to speculate that the C2 NFLE-mutant residues may also alter channel properties and this may explain their enhancement of ACh-induced peak function (Figure 3) and per-receptor function (especially in HS-isoform preferring expression systems; Figure 7D and 7E). Single channel studies are beyond the scope of the present work, but could be valuable in probing the changes in unitary receptor properties that underlie our macroscopic-current observations. Certainly, findings from this study and one previously-published manuscript (McClure-Begley et al., 2013) indicate that residues in this part of the cytoplasmic loop play important roles in regulating nAChR functional properties and isoform ratios.
4.6 Conclusions
The current study functionally characterized two novel and little-studied C2 NFLE-associated mutations. We find for the first time that the two C2 NFLE-associated mutant subunits do indeed produce significant functional effects when incorporated into α4β2-nAChR. Comparing the functional consequences of these C2 mutations to those of the TM-mutants may further illuminate the bases for alterations in receptor function and expression that contribute to AD/NFLE disease pathology. For example, C2-domain NFLE-associated mutants did not produce shifts in ACh or other agonist EC50 values at either of the HS- or LS- α4β2-nAChR isoform populations, as commonly seen for TM-domain ADNFLE mutant subunits. However, the consistently-enhanced proportion of functional HS-isoform α4β2-nAChR expressed, and enlarged overall macroscopic current responses to ACh, lead to enhanced function in response to low levels of ACh. This is a common outcome shared with the previously-characterized TM-domain mutants, although it results from different underlying changes in macroscopic nAChR behavior. The fact that this outcome is retained across AD/NFLE-associated α4 and β2 nAChR subunit variants, found in different subunit domains, suggests that it may be especially critical to AD/NFLE etiology. Our findings therefore reinforce models that postulate enhancement in neuronal excitability initiates an imbalance between inhibitory and excitatory synaptic transmission, leading to seizures (Klaassen et al., 2006; Rodrigues-Pinguet et al., 2005). It is also notable that both C2- and TM- domain NFLE mutant subunits alter the HS-to-LS isoform ratio (although in different directions). Such changes per se potentially could also initiate imbalances between inhibitory and excitatory synaptic transmission.
In addition to confirming for the first time functional effects of NFLE-linked C2-mutant nAChR subunits, and focusing our understanding of the nAChR properties most relevant to AD/NFLE etiology, this study also highlights the importance of C2 loop residues in regulating nAChR properties. Evidence presented here indicates that residues in the cytoplasmic loop section adjacent to TM3 can significantly influence agonist-induced peak current magnitudes, relative efficacies of agonists, cell-surface isoform expression ratios and overall receptor cell-surface expression levels.
Highlights.
Unlike α4β2 nAChR ADNFLE TM-domain mutations, C2 NFLE-associated mutations do not alter ACh potency.
C2 NFLE-associated mutations significantly enhance ACh-induced peak currents in both the α4β2 nAChR HS- and LS- isoforms.
C2 NFLE-associated mutations favor the expression of the α4β2 HS-isoform.
The β2(V337G) C2 NFLE-associated mutation significantly reduced receptor cell surface expression.
The C2 mutations significantly enhanced the function per unit of receptor when expressed in the α4β2 nAChR HS-isoform.
Acknowledgments
The authors would like to thank John F. Cooper (Department of Neuroscience, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA) for preparation of the [125I]-labeled monoclonal antibodies used in this study, and for his guidance in their use. Andrew A. George, Linda M. Lucero and Trellanie W. Jones (all at the Barrow Neurological Institute, Phoenix, AZ) provided assistance in preparation of this manuscript, we thank them for their attention. We would also like to thank Dr. Roger L. Papke (University of Florida School of Medicine, Gainesville, FL) for his extremely valuable comments and suggestions.
Role of Funding Sources: Research was financially supported by NIH [Grant R21 DA026627], Barrow Neurological Foundation Start-up Funds and the Barrow Neurological Foundation Fellowship [Grant 45GTS5003031882]. These funding agencies had no other involvement in this research.
Nonstandard Abbreviations
- nAChR
nicotinic acetylcholine receptor
- NFLE
nocturnal frontal lobe epilepsy
- ADNFLE
autosomal dominant nocturnal frontal lobe epilepsy
- HS
high sensitivity
- LS
low sensitivity
- TEVC
two-electrode voltage clamp
- ACh
acetylcholine
- TM
transmembrane
- C2
intracellular cytoplasmic loop
- DHβE
dihydro-β-erythroidine hydrobromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article: Acetylcholine chloride (PubChem CID: 6060), (−)-nicotine hydrogen tartrate salt (PubChem CID: 89594), cytisine (PubChem CID: 10235), sazetidine-A (PubChem CID: 11983356), Dihydro-β-erythroidine (PubChem CID: 31762) and mecamylamine hydrochloride (PubChem CID: 13221).
- Participated in research design, data analysis and interpretation: Weltzin, Whiteaker.
- Wrote or contributed to the writing of the manuscript: Weltzin, Whiteaker, Lukas, Lindstrom.
- Final approval of the version to be submitted: All authors.
Contributor Information
Maegan M. Weltzin, Email: maeganweltzin@gmail.com.
Jon M. Lindstrom, Email: jslkk@mail.med.upenn.edu.
Ronald J. Lukas, Email: Ronald.Lukas@dignityhealth.org.
Paul Whiteaker, Email: Paul.Whiteaker@dignityhealth.org.
References
- Bertrand D, Elmslie F, Hughes E, Trounce J, Sander T, Bertrand S, Steinlein OK. The CHRNB2 mutation I312M is associated with epilepsy and distinct memory deficits. Neurobiol Dis. 2005;20:799–804. doi: 10.1016/j.nbd.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Picard F, Le Hellard S, Weiland S, Favre I, Phillips H, Bertrand S, Berkovic SF, Malafosse A, Mulley J. How mutations in the nAChRs can cause ADNFLE epilepsy. Epilepsia. 2002;43(Suppl 5):112–122. doi: 10.1046/j.1528-1157.43.s.5.16.x. [DOI] [PubMed] [Google Scholar]
- Briggs CA, Gubbins EJ, Putman CB, Thimmapaya R, Meyer MD, Surowy CS. High- and low-sensitivity subforms of α4β2 and α3β2 nAChRs. J Mol Neurosci. 2006;30:11–12. doi: 10.1385/JMN:30:1:11. [DOI] [PubMed] [Google Scholar]
- Brodtkorb E, Picard F. Tobacco habits modulate autosomal dominant nocturnal frontal lobe epilepsy. Epilepsy Behav. 2006;9:515–520. doi: 10.1016/j.yebeh.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Carbone AL, Moroni M, Groot-Kormelink PJ, Bermudez I. Pentameric concatenated (α4)(2)(β2)(3) and (α4)(3)(β2)(2) nicotinic acetylcholine receptors: subunit arrangement determines functional expression. Br J Pharmacol. 2009;156:970–981. doi: 10.1111/j.1476-5381.2008.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu L, Fang Y, He Z, Peng B, Shen Y, Xu Q. A novel mutation of the nicotinic acetylcholine receptor gene CHRNA4 in sporadic nocturnal frontal lobe epilepsy. Epilepsy Res. 2009;83:152–156. doi: 10.1016/j.eplepsyres.2008.10.009. [DOI] [PubMed] [Google Scholar]
- De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, Ballabio A, Wanke E, Casari G. The nicotinic receptor β2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet. 2000;26:275–276. doi: 10.1038/81566. [DOI] [PubMed] [Google Scholar]
- Eaton JB, Lucero LM, Stratton H, Chang Y, Cooper JF, Lindstrom JM, Lukas RJ, Whiteaker P. The Unique α4(+)/(−)α4 Agonist Binding Site in (α4)3(β2)2 Subtype Nicotinic Acetylcholine Receptors Permits Differential Agonist Desensitization Pharmacology versus the (α4)2(β2)3 Subtype. J Pharmacol Exp Ther. 2014;348:46–58. doi: 10.1124/jpet.113.208389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figl A, Viseshakul N, Shafaee N, Forsayeth J, Cohen BN. Two mutations linked to nocturnal frontal lobe epilepsy cause use-dependent potentiation of the nicotinic ACh response. J Physiol. 1998;513(Pt 3):655–670. doi: 10.1111/j.1469-7793.1998.655ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor α4 or β2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of α4 and β2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Hales TG, Dunlop JI, Deeb TZ, Carland JE, Kelley SP, Lambert JJ, Peters JA. Common determinants of single channel conductance within the large cytoplasmic loop of 5-hydroxytryptamine type 3 and α4β2 nicotinic acetylcholine receptors. J Biol Chem. 2006;281:8062–8071. doi: 10.1074/jbc.M513222200. [DOI] [PubMed] [Google Scholar]
- Harpsoe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci. 2011;31:10759–10766. doi: 10.1523/JNEUROSCI.1509-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Hirose S, Iwata H, Akiyoshi H, Kobayashi K, Ito M, Wada K, Kaneko S, Mitsudome A. A novel mutation of CHRNA4 responsible for autosomal dominant nocturnal frontal lobe epilepsy. Neurol. 1999;53:1749–1753. doi: 10.1212/wnl.53.8.1749. [DOI] [PubMed] [Google Scholar]
- Hoda JC, Gu W, Friedli M, Phillips HA, Bertrand S, Antonarakis SE, Goudie D, Roberts R, Scheffer IE, Marini C, Patel J, Berkovic SF, Mulley JC, Steinlein OK, Bertrand D. Human nocturnal frontal lobe epilepsy: pharmocogenomic profiles of pathogenic nicotinic acetylcholine receptor beta-subunit mutations outside the ion channel pore. Mol Pharmacol. 2008;74:379–391. doi: 10.1124/mol.107.044545. [DOI] [PubMed] [Google Scholar]
- Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J. Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci U S A. 2006;103:19152–19157. doi: 10.1073/pnas.0608215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracun S, Harkness PC, Gibb AJ, Millar NS. Influence of the M3–M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br J Pharmacol. 2008;153:1474–1484. doi: 10.1038/sj.bjp.0707676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YP, Xu L, Eaton JB, Zhao L, Wu J, Lukas RJ. Roles for nicotinic acetylcholine receptor subunit large cytoplasmic loop sequences in receptor expression and function. J Pharmacol Exp Ther. 2005;314:455–466. doi: 10.1124/jpet.105.084954. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Gerzanich V, Nelson M, Olale F, Lindstrom J. Mutation causing autosomal dominant nocturnal frontal lobe epilepsy alters Ca2+ permeability, conductance, and gating of human α4β2 nicotinic acetylcholine receptors. J Neurosci. 1997;17:9035–9047. doi: 10.1523/JNEUROSCI.17-23-09035.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Lindstrom J. Expression of Functional Human α6β2β3* Acetylcholine Receptors in Xenopus laevis Oocytes Achieved through Subunit Chimeras and Concatamers. Mol. Pharmacol. 2011;79:126–140. doi: 10.1124/mol.110.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment decreases striatal α6 nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol. 2005;67:1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Leonardi M, Ustun TB. The global burden of epilepsy. Epilepsia. 2002;43(Suppl 6):21–25. doi: 10.1046/j.1528-1157.43.s.6.11.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Lu C, Li Z, Zhou S, Li X, Ji L, Lu Q, Lv R, Wu L, Ma X. The identification of a novel mutation of nicotinic acetylcholine receptor gene CHRNB2 in a Chinese patient: Its possible implication in non-familial nocturnal frontal lobe epilepsy. Epilepsy Res. 2011;95:94–99. doi: 10.1016/j.eplepsyres.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the β2 subunit. J Pharmacol Exp Ther. 1999;289:1090–1103. [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, Bermudez I. Additional acetylcholine (ACh) binding site at α4/α4 interface of (α4β2)2α4 nicotinic receptor influences agonist sensitivity. J Biol Chem. 2011;286:31043–31054. doi: 10.1074/jbc.M111.262014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure-Begley TD, Stone KL, Marks MJ, Grady SR, Colangelo CM, Lindstrom JM, Picciotto MR. Exploring the nicotinic acetylcholine receptor-associated proteome with iTRAQ and transgenic mice. Genomics Proteomics Bioinformatics. 2013;11:207–218. doi: 10.1016/j.gpb.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, Bermudez I. Stoichiometry and pharmacology of two human α4β2 nicotinic receptor types. J Mol Neurosci. 2006;30:95–96. doi: 10.1385/JMN:30:1:95. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. α4β2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006a;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. α4β2 nicotinic receptors with high and low acetylcholine sensitivity: Pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006b;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Motamedi GK, Lesser RP. Autosomal dominant nocturnal frontal lobe epilepsy. Adv Neurol. 2002;89:463–473. [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Phillips HA, Scheffer IE, Berkovic SF, Hollway GE, Sutherland GR, Mulley JC. Localization of a gene for autosomal dominant nocturnal frontal lobe epilepsy to chromosome 20q 13.2. Nat Genet. 1995;10:117–118. doi: 10.1038/ng0595-117. [DOI] [PubMed] [Google Scholar]
- Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schollhorn-Peyronneau MA, Roumenov D, Brodtkorb E, Zuberi S, Gambardella A, Steinborn B, Hufnagel A, Valette H, Bottlaender M. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129:2047–2060. doi: 10.1093/brain/awl156. [DOI] [PubMed] [Google Scholar]
- Pollock VV, Pastoor T, Katnik C, Cuevas J, Wecker L. Cyclic AMP-dependent protein kinase A and protein kinase C phosphorylate α4β2 nicotinic receptor subunits at distinct stages of receptor formation and maturation. Neurosci. 2009;158:1311–1325. doi: 10.1016/j.neuroscience.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provini F, Plazzi G, Tinuper P, Vandi S, Lugaresi E, Montagna P. Nocturnal frontal lobe epilepsy. A clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(Pt 6):1017–1031. doi: 10.1093/brain/122.6.1017. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Pinguet N, Jia L, Li M, Figl A, Klaassen A, Truong A, Lester HA, Cohen BN. Five ADNFLE mutations reduce the Ca2+ dependence of the mammalian α4β2 acetylcholine response. J Physiol. 2003;550:11–26. doi: 10.1113/jphysiol.2003.036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Pinguet NO, Pinguet TJ, Figl A, Lester HA, Cohen BN. Mutations linked to autosomal dominant nocturnal frontal lobe epilepsy affect allosteric Ca2+ activation of the α4β2 nicotinic acetylcholine receptor. Mol Pharmacol. 2005;68:487–501. doi: 10.1124/mol.105.011155. [DOI] [PubMed] [Google Scholar]
- Scheffer IE, Bhatia KP, Lopes-Cendes I, Fish DR, Marsden CD, Andermann E, Andermann F, Desbiens R, Keene D, Cendes F, et al. Autosomal dominant nocturnal frontal lobe epilepsy. A distinctive clinical disorder. Brain. 1995;118(Pt 1):61–73. doi: 10.1093/brain/118.1.61. [DOI] [PubMed] [Google Scholar]
- Scheffer IE, Bhatia KP, Lopes-Cendes I, Fish DR, Marsden CD, Andermann F, Andermann E, Desbiens R, Cendes F, Manson JI, et al. Autosomal dominant frontal epilepsy misdiagnosed as sleep disorder. Lancet. 1994;343:515–517. doi: 10.1016/s0140-6736(94)91463-x. [DOI] [PubMed] [Google Scholar]
- Son CD, Moss FJ, Cohen BN, Lester HA. Nicotine normalizes intracellular subunit stoichiometry of nicotinic receptors carrying mutations linked to autosomal dominant nocturnal frontal lobe epilepsy. Mol Pharmacol. 2009;75:1137–1148. doi: 10.1124/mol.108.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5:400–408. doi: 10.1038/nrn1388. [DOI] [PubMed] [Google Scholar]
- Steinlein OK. Animal models for autosomal dominant frontal lobe epilepsy: on the origin of seizures. Expert Rev Neurother. 2010;10:1859–1867. doi: 10.1586/ern.10.130. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Hoda JC, Bertrand S, Bertrand D. Mutations in familial nocturnal frontal lobe epilepsy might be associated with distinct neurological phenotypes. Seizure. 2012a;21:118–123. doi: 10.1016/j.seizure.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Kaneko S, Hirose S. Nicotinic acetylcholine receptor mutations. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. Bethesda (MD): 2012b. [PubMed] [Google Scholar]
- Steinlein OK, Magnusson A, Stoodt J, Bertrand S, Weiland S, Berkovic SF, Nakken KO, Propping P, Bertrand D. An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Hum Mol Genet. 1997;6:943–947. doi: 10.1093/hmg/6.6.943. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, Scheffer IE, Berkovic SF. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–203. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- Stokes C, Treinin M, Papke RL. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2015;36:514–523. doi: 10.1016/j.tips.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (α4)3(β2)2 stoichiometry greatly exceeds that of (α4)2(β2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Tsetlin V, Kuzmin D, Kasheverov I. Assembly of nicotinic and other Cys-loop receptors. J Neurochem. 2011;116:734–741. doi: 10.1111/j.1471-4159.2010.07060.x. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Weiland S, Witzemann V, Villarroel A, Propping P, Steinlein O. An amino acid exchange in the second transmembrane segment of a neuronal nicotinic receptor causes partial epilepsy by altering its desensitization kinetics. FEBS Lett. 1996;398:91–96. doi: 10.1016/s0014-5793(96)01215-x. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Cooper JF, Salminen O, Marks MJ, McClure-Begley TD, Brown RWB, Collins AC, Lindstrom JM. Immunolabeling demonstrates the interdependence of mouse brain α4 and β2 nicotinic acetylcholine receptor subunit expression. J Comp Neurol. 2006;499:1016–1038. doi: 10.1002/cne.21181. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Wilking JA, Brown RW, Brennan RJ, Collins AC, Lindstrom JM, Boulter J. Pharmacological and immunochemical characterization of α2* nicotinic acetylcholine receptors (nAChRs) in mouse brain. Acta Pharmacol Sin. 2009;30:795–804. doi: 10.1038/aps.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci. 1988;8:3395–3404. doi: 10.1523/JNEUROSCI.08-09-03395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby JO, Pope KJ, Eaton V. Nicotine as an antiepileptic agent in ADNFLE: an N-of-one study. Epilepsia. 2003;44:1238–1240. doi: 10.1046/j.1528-1157.2003.58102.x-i1. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes α4β2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine-A is a potent and selective agonist at native and recombinant α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]