Abstract
OBJECTIVE
Osteonecrosis is a potential complication of glucocorticoid chemotherapy in children surviving leukemia. Early diagnosis may allow effective interventions to minimize or ameliorate joint deterioration and obviate surgical intervention. We investigated the significance of MRI signal changes that precede the currently recognized “double-line” changes, which are considered pathognomic of osteonecrosis.
MATERIALS AND METHODS
We retrospectively reviewed MRI scans acquired during prospective screening and follow-up of pediatric patients with leukemia for osteonecrosis.
RESULTS
Of 481 patients, we identified 21 cases (4.3%; 12 boys; median age at leukemia diagnosis, 12.8 years) with subtle poorly defined geographically delineated MRI signal abnormalities in knees or hips, or both, that progressed over a median of 4 months (range, 1.6–18.5 months) to florid MRI signs of osteonecrosis. Articular surface collapse developed in three hips (two patients) and three knees (three patients). Three patients subsequently underwent surgical intervention (one bilateral total hip arthroplasty and one bilateral and one unilateral hip core decompression). The median duration of follow-up was 27 months (range, 1.9–90.7 months).
CONCLUSION
The MRI signal abnormalities described here appear to herald extensive osteonecrosis and precede the typical MRI findings of osteonecrosis previously reported in the literature.
Keywords: articular collapse, MRI, osteonecrosis, signal abnormalities
Despite their contribution to survival rates exceeding 90% in children with acute lymphoblastic leukemia (ALL) [1], treatment regimens that include high-dose glucocorticoids have increased the incidence of long-term complications such as osteonecrosis. Osteonecrosis has been reported in 38% of children with ALL who have undergone chemotherapy [2] and in as many as 44% of children who have undergone allogeneic bone marrow transplantation [3]. Early-stage osteonecrosis responds favorably to conservative treatment or minimally invasive surgery [4–6]. Failure to detect early-stage osteonecrosis is associated with complications such as articular collapse and secondary arthritis that lead to significant joint deterioration, for which adjustment of chemotherapy and surgical interventions (e.g., core decompression, resurfacing arthroplasty, or total joint arthroplasty) are the only treatment options [4, 6, 7]. Early diagnosis allows prompt intervention directed at preserving the joint and reducing the need for aggressive surgical intervention [4, 8].
MRI can detect bone ischemia and osteonecrosis at an early stage, when results of most other imaging modalities are negative and when the patient is still asymptomatic [7, 9]. MRI is nearly 100% sensitive and specific for early osteonecrosis [8] and is, therefore, highly effective for screening without exposing patients to ionizing radiation. At our institution, all patients with ALL and all recipients of bone marrow transplantations undergo MRI screening for early detection of osteonecrosis, regardless of symptoms. MRI features currently considered typical of osteonecrosis are well-circumscribed geographic areas with margins of low signal on T1-weighted and high signal on T2-weighted images (viable tissue) or with low signal on both T1-weighted and T2-weighted images (necrotic tissue) [9–11]. The “double-line” sign, consisting of an outer low signal and inner high signal circumscribing the area on T2-weighted sequences is also considered diagnostic of osteonecrosis [10].
Standardized prospective MRI screening of children with ALL and of those who have undergone allogeneic bone marrow transplantation has allowed us to observe subtle signal changes in knees and hips that precede the earliest reported MRI signs of osteonecrosis and also appear to predict subsequent development of extensive osteonecrosis on follow-up MRI examinations. These changes include a thin indistinct single winding line of T1 hypointensity with a corresponding T2 STIR hyperintensity marginating discrete areas of normal marrow signal in epiphysis, metaphyses, or diaphyses. We hypothesized that these lesions indicated an earlier stage of osteonecrosis than is currently recognized on MRI [8, 10].
We retrospectively reviewed the MRI studies of patients with ALL who had been prospectively screened for osteonecrosis to investigate the significance of these signal abnormalities, their outcome, and risk factors for their development.
Materials and Methods
Patients
The patient cohort was identified through electronic medical records. After approval by the St. Jude institutional review board, we reviewed the prospectively acquired MRI screening studies of hips and knees of 481 patients with ALL performed between 2001 and 2011. MRI reviews were performed as part of screening incorporated within treatment protocols for ALL and bone marrow transplantation. Information about treatment, diagnosis, and demographics was extracted from medical records. All data were managed in accordance with HIPAA regulations.
Of the 481 patients, 391 were treated according to the institutional Total Therapy XV protocol [12], 55 were treated according to the Total Therapy XVI protocol [11], and another 34 underwent allogenic bone marrow transplantation after leukemia therapy (Total Therapy XV, n = 33; Total Therapy XVI, n = 1). One patient initially treated according to the COG9061 protocol at an affiliated hospital underwent allogenic bone marrow transplantation for recurrent ALL at our institution 14 months after completing chemotherapy. All chemotherapy protocols included a standard immunosuppressive regimen of high-dose corticosteroids.
MRI Evaluation
MRI screening was performed on a 1.5-T (Avanto, Siemens Healthcare) or a 3-T (Trio, Siemens Healthcare) scanner. Patients treated according to the Total Therapy XV and XVI protocols were screened for osteonecrosis after each reinduction chemotherapy phase (weeks 12–14 and 22–24 of treatment) regardless of symptoms. Additional follow-up MRI studies were largely dependent on results at screening examination or the development of clinical symptoms. Patients who were symptomatic or positive for osteonecrosis on MRI screening were referred for clinical evaluation, where needs for further assessment or potential interventions were determined and individualized according to clinical symptoms, MRI findings, or response to osteonecrotic treatment. Patients treated with bone marrow transplantation had a baseline MRI screening at the time of transplantation and follow-up studies annually, thereafter. No further studies were done if the second follow-up MRI examination was negative and the patient remained asymptomatic. The presence of symptoms or a positive second follow-up examination warranted further imaging studies until symptoms resolved or imaging findings peaked. The one patient who had received primary treatment elsewhere was screened 3 months after undergoing transplantation at our institution. MRI screening examinations included imaging of bilateral hips and knees with unenhanced coronal T1-weighted and STIR imaging plus a sagittal 2D FLASH sequence.
Our cohort selection was based on the presence of MRI signal abnormalities on the first screening study, which were identified during protocol-driven review by a single experienced pediatric radiologist who had no knowledge of clinical findings. The signal characteristics of interest were a thin indistinct single winding line of T1 hypointensity with a corresponding T2 STIR hyperintensity or marginating discrete areas of normal marrow signal in the epiphysis, metaphyses, or diaphyses of the examined bones (Figs. 1A and 1B). To determine whether these signal abnormalities evolved over time to more familiar MRI patterns of osteonecrosis, including sharp thick serpiginous lines of low signal on T1-weighted and high signal on T2-weighted images (viable tissue) or with low signal on both T1-weighted and T2-weighted images (necrotic tissue) marginating geographic areas of bone marrow altered peripherally by accompanying marrow edema [9–11], we retrospectively reviewed the follow-up MRI studies of all patients who showed the early signal characteristics of interest.
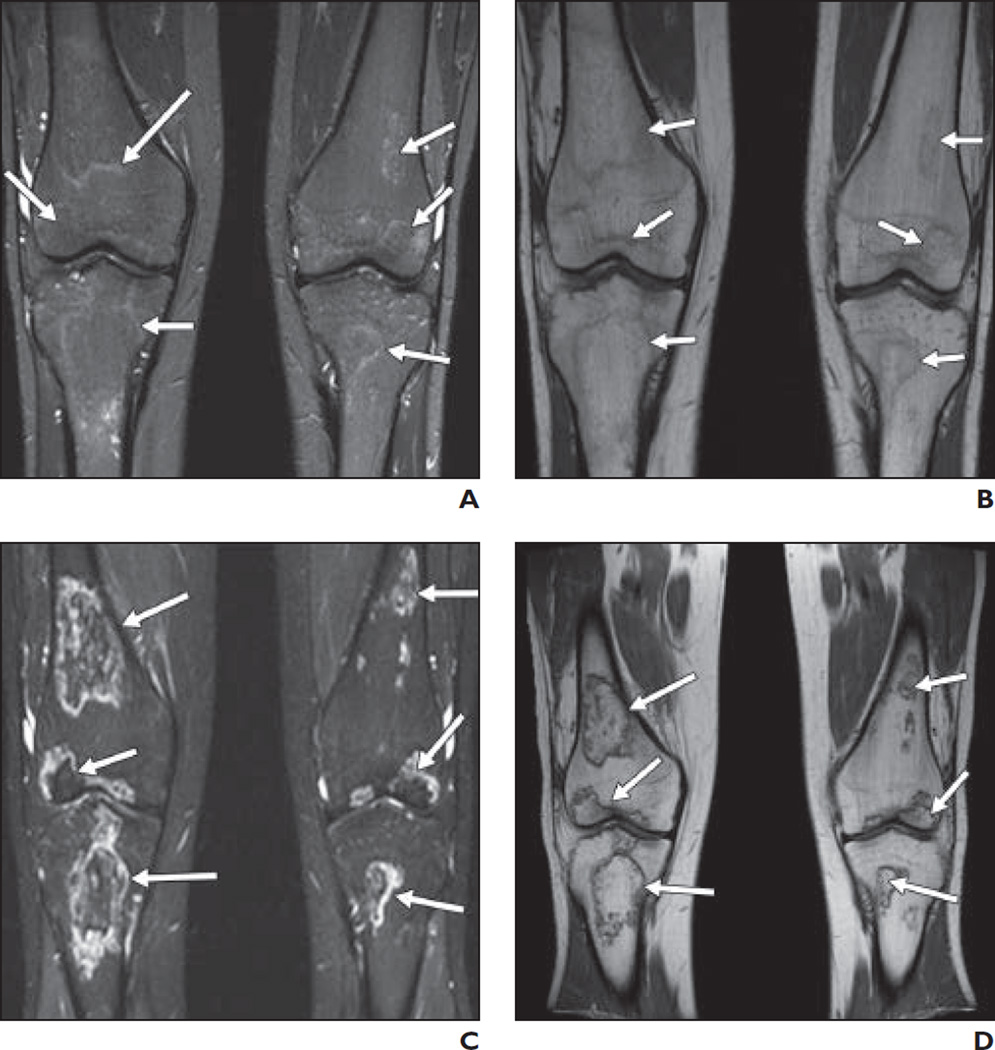
Fig. 1. 17-year-old boy.
A and B, On STIR (A) and T1-weighted (B) coronal images of knees, signal changes (arrows) are seen in distal femoral epiphyses (> 30% articular involvement), proximal tibial epiphyses, and metaphyses bilaterally.
C and D, STIR (C) and T1-weighted (D) coronal images of knees at 3-month follow-up examination show evolution of previous signals into typical osteonecrotic lesions (arrows) conforming to original signal pattern.
Because adolescents are reported to experience a higher prevalence and severity of osteonecrosis [13, 14], we classified all patients as having open or closed physes. In addition, because 30% or more of osteonecrotic involvement of the epiphysial articular surface is associated with adverse prognosis and outcome [13–16], we classified patients as showing less than 30%, 30% or higher, or no MRI signal changes involving the epiphysial articular surfaces of the hips and knees. This classification allowed us to analyze whether the epiphysial articular signal changes observed in our cohort were predictive of articular collapse or the need for surgical intervention. One patient who had signal changes limited to proximal femoral metaphyses bilaterally was excluded from this analysis.
Results
Patients
Twenty-one of the 481 patients (4.3%) had atypical MRI signal changes in the hips or knees, or both, on MRI screening studies (Total Therapy XV, n = 16; Total Therapy XVI, n = 4; COG9061 followed by allogeneic bone marrow transplantation, n = 1). These 21 patients included 12 (57%) boys. The median age at the time of diagnosis of ALL was 11.8 years (range, 7.5–18.7 years). The mean age at the time of the first MRI screening examination was 13 years (range, 4–19 years). The median time from diagnosis to the most recent follow-up examination was 28.1 months (range, 2.5–90.7 months; Table 1).
TABLE 1.
Summary of Demographics, Joint and Regional Bone Involvement, and Outcomes of 20 Patients With Described Signal Abnormalities
| Patient No., Study |
Sex | Age (y) at First MRI Examination |
Time Between Diagnosis and First MRI Examination (mos) |
Time Between First and Second MRI Examination (mos) |
Time Between Diagnosis and Follow-Up Examination (mos) |
Joint | Epiphyseal | Metaphyseal | Diaphyseal | Unilateral vs Bilateral |
Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 30% | < 30% | None | |||||||||||
| 1 | Male | 12.2 | 5.1 | 13.8 | 90.7 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Positive | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Stable osteonecrosis | ||||||
| 1 | Male | 12.2 | 5.1 | 13.8 | 90.7 | Hips | |||||||
| MRI 1 | Positive | Negative | Negative | Negative | Negative | Bilateral | Extensive osteonecrosis with collapse | ||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Total hip replacement | ||||||
| 2 | Male | 14 | 6.2 | 1.6 | 73.5 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis with partial collapse | ||||||
| 3 | Male | 13.4 | 5.8 | 3.3 | 31.6 | Knees | |||||||
| MRI 1 | Negative | Negative | Positive | Positive | Negative | Bilateral | |||||||
| MRI 2 | Negative | Positive | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis | ||||||
| 4 | Female | 11.9 | 4.4 | 2.8 | 48.3 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis | ||||||
| 5 | Female | 14.9 | 3 | 2.5 | 2.5 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Negative | Bilateral | Stable osteonecrosis | ||||||
| 6 | Male | 18 | 8.3 | 18.5 | 19.3 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Unilateral | Extensive osteonecrosis | ||||||
| 7 | Male | 19.2 | 5.7 | 3.6 | 33.1 | Knees | |||||||
| MRI 1 | Negative | Negative | Positive | Positive | Positive | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis with collapse | ||||||
| 8 | Female | 11 | 5.9 | 3 | 25 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Negative | Bilateral | Stable osteonecrosis | ||||||
| 9 | Male | 17 | 6 | 3.2 | 38.8 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Negative | Bilateral | Stable osteonecrosis with collapse | ||||||
| 10 | Female | 11.3 | 5.9 | 3.2 | 37.6 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Positive | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Stable osteonecrosis | ||||||
| 11 | Male | 15.2 | 7.5 | 3.2 | 23.5 | Knees | |||||||
| MRI 1 | Negative | Positive | Negative | Positive | Positive | Bilateral | |||||||
| MRI 2 | Negative | Positive | Negative | Positive | Positive | Bilateral | Stable osteonecrosis | ||||||
| 12 | Female | 15.8 | 5.9 | 2.5 | 24.9 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Negative | Negative | Unilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis | ||||||
| 13 | Male | 16.7 | 5.8 | 3 | 31.1 | Knees | |||||||
| MRI 1 | Negative | Positive | Negative | Negative | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis | ||||||
| 14 | Male | 11.8 | 7 | 3.9 | 15.8 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Negative | Bilateral | Stable osteonecrosis | ||||||
| 15 | Female | 10.3 | 6.3 | 2.5 | 8.7 | Knees | |||||||
| MRI1 | Negative | Positive | Negative | Positive | Positive | Bilateral | |||||||
| MRI2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis | ||||||
| 15 | Female | 10.3 | 6.3 | 2.5 | 8.7 | Hips | |||||||
| MRI 1 | Negative | Positive | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis with collapse | ||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Right hip core decompression | ||||||
| 16 | Female | 12 | 5.9 | 3.4 | 3.4 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Negative | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis | ||||||
| 17 | Male | 8.7 | 7 | 1.9 | 1.9 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Positive | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Stable osteonecrosis | ||||||
| 18 | Male | 4.1 | 6.5 | 2.8 | 32 | Knees | |||||||
| MRI 1 | Negative | Negative | Positive | Positive | Negative | Unilateral | |||||||
| MRI 2 | Negative | Negative | Positive | Positive | Negative | Unilateral | Stable osteonecrosis | ||||||
| 19 | Male | 12.7 | 6 | 2.5 | 2.5 | Knees | |||||||
| MRI 1 | Negative | Positive | Negative | Positive | Positive | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Extensive osteonecrosis | ||||||
| 20 | Female | 17.7 | 6 | 2.3 | 7 | Knees | |||||||
| MRI 1 | Positive | Negative | Negative | Positive | Positive | Bilateral | |||||||
| MRI 2 | Positive | Negative | Negative | Positive | Positive | Bilateral | Stable osteonecrosis | ||||||
| 20 | Female | 17.7 | 6 | 2.3 | 7 | Hips | |||||||
| MRI 1 | Negative | Negative | Positive | Negative | Positive | Bilateral | |||||||
| MRI 2 | Negative | Positive | Negative | Negative | Positive | Bilateral | Extensive osteonecrosis | ||||||
MRI Findings
The first MRI screening examination was performed a mean of 6.7 months (range, 5–28 months) after the start of chemotherapy (3 months after allogenic bone marrow transplantation in one patient). The involved joints were skeletally immature in 15 of the 21 patients (71%) showing MRI signal changes and showed complete or nearly complete closure of the epiphysial plates in six patients (28.5%). Bilateral knee involvement occurred in 18 (85.7%) patients, and single-knee involvement was present in two patients. Three of the patients with bilateral knee involvement also showed bilateral involvement of the hips. One patient had involvement of both hips without involvement of the knees.
Twenty patients with knee involvement had signal changes showing 30% or higher (n = 13) (Figs. 1A and 1B) and less than 30% (n = 4) epiphysial articular surface involvement. There were three cases with signal changes limited to the metaphyses (n = 2) and metaphyses and diaphyses (n = 1) with no epiphysial articular surface involvement on screening MRI examination (Figs. 2A and 2B). Of the three patients with signal changes in the hip joints, one had 30% or greater epiphysial articular involvement (Fig. 3), one had less than 30%, and one had only diaphysial signal changes with no epiphysial articular surface involvement.
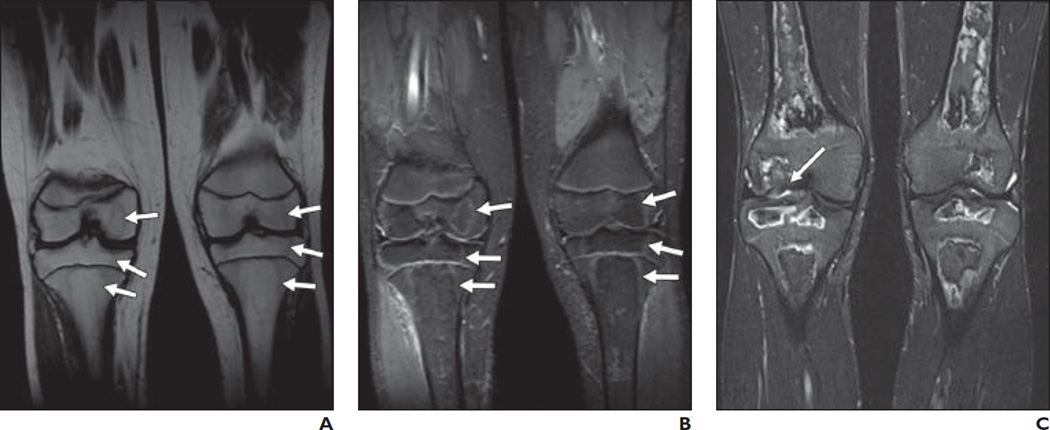
Fig. 2. 10-year-old girl.
A and B, On STIR (A) and T1-weighted (B) coronal images of knees, subtle signal changes (arrows) involving tibial metaphyses and femoral diaphyses bilaterally are seen.
C and D, STIR (C) and T1-weighted (D) coronal images of knees at 2.5-month follow-up examination show osteonecrosis (arrows) involving epiphyses, metaphyses, and diaphyses more extensively in location and size than that predicted by initial signal changes.
Fig. 3. 12-year-old boy.
A and B, On T1-weighted (A) and STIR (B) coronal images of hips, signal changes (arrows) involving > 30% femoral epiphyseal articular surface bilaterally are seen.
C and D, T1-weighted (C) and STIR (D) images show articular collapse (arrows) of femoral heads bilaterally on follow-up MRI performed 2.5 years later.
Follow-Up
The mean interval between the screening and the first follow-up MRI examinations was 4 months (range, 1.6–18.5 months). The mean follow-up duration after the screening MRI study was 28 months (range, 3–91 months), and patients had a mean of four MRI studies (range, 1–8 studies). One patient with bilateral signal changes involving only the proximal femoral metaphyses on screening MRI had no follow-up studies.
Review of the first follow-up MRI examination of the remaining 20 patients revealed evolution of the signal changes to those typical of osteonecrosis; these findings were interpreted as osteonecrosis in all 20 patients (Figs. 1B and 1D). In half of the 20 patients with knee signal abnormalities and all of the three with hip signal abnormalities, osteonecrosis extended beyond the original pattern of signal distribution (Figs. 2B and 2D); three of these patients subsequently developed MRI features of collapse of the articular surface of the knees, and two developed collapse of the articular surface of the hip (bilateral collapse in one).
Knees With 30% or Greater Epiphysial Articular Surface Signal Abnormality: 13 Cases
Eight patients had 30% or greater epiphysial articular surface and metaphysial signal involvement of knee joints on the first screening MRI. On the first follow-up examination, four of these patients showed a pattern of osteonecrosis that was superimposable (in size and location) on the original signal pattern (Figs. 1C and 1D). One of these patients developed MRI features of articular collapse of the knee joint 2.8 years after the first screening MRI examination (Fig. 4C). In the remaining four patients, the osteonecrotic lesions that developed were larger than the original signal pattern and extended into the diaphyses; one of these patients developed MRI features of knee articular collapse 6 years after the first screening study. One patient whose initial signal changes involved only the epiphysial articular surface (≥ 30%) of one knee and showed no metaphysial or diaphysial signal changes progressed to osteonecrotic lesions in the metaphyses, diaphyses, and one epiphysis (≥ 30%) of both knees found on follow-up MRI. In the four cases with 30% or greater epiphysial articular plus metaphysial and diaphysial signal involvement, the lesions remained stable in appearance and size on follow-up studies.
Fig. 4. 14-year-old boy.
A and B, On T1-weighted (A) and STIR (B) coronal images of knees, signal changes (arrows) involving > 30% epiphyseal articular surfaces of tibial and femoral condyles are seen bilaterally.
C, STIR coronal knee image on follow-up MRI performed 6 years later shows articular cartilage irregularity (arrow) involving right femoral condyle.
Knees With Less Than 30% Epiphysial Articular Surface Signal Abnormality: Four Cases
Three patients had less than 30% epiphysial articular surface, with varying degrees of metaphysial and diaphysial signal changes. Two of these patients experienced progression to osteonecrosis involving 30% or more of the epiphysial articular surface with further expansion of the metaphysial and diaphysial lesions, whereas one patient had osteonecrosis confined to the original signal pattern. One patient who had less than 30% epiphysial signal involvement as the only abnormality on the first screening MRI examination developed osteonecrosis extending into the metaphyses, diaphysis, and epiphyses (≥ 30%) on the follow-up study. None of these patients experienced articular collapse.
Knees Showing No Epiphysial Articular Surface Signal Abnormality: Three Cases
In three patients, signal changes on first screening MRI examination were present only in the metaphyses (n = 2) or in metaphysis and diaphyses (n = 1) (Figs. 2A and 2B). Of the two patients with signal changes initially limited to the metaphysis, one progressed to osteonecrosis involving less than 30% of the epiphysis with expansion of lesions in the metaphysis and extension into the diaphysis, whereas the other patient’s lesions remained stable in size and distribution (limited to the metaphyses) at follow-up. The patient with metaphysial and diaphysial signal changes showed 30% or greater epiphysial articular osteonecrosis (Figs. 4B and 4C) with MRI collapse of the knee articular surface 3 years after the first screening MRI study.
Hips With and Without Epiphysial Articular Involvement: Three Cases
The patient who had 30% or greater epiphysial articular surface involvement of the hips showed progressive flattening of the femoral heads on follow-up studies, with complete MRI evidence of collapse of both femoral heads 2.5 years after the first screening study (Figs. 3C and 3D) followed by bilateral total hip replacement 6 months later. One patient with less than 30% involvement of the epiphyses showed 30% or higher involvement with gradual flattening of the right femoral head on follow-up. This patient underwent right hip core decompression 11 months after the first screening MRI examination. The patient who had only diaphysial signal changes and no epiphysial involvement showed progression to less than 30% epiphysial involvement on follow-up.
Discussion
MRI features currently considered typical of early osteonecrosis are described as well-circumscribed geographic areas with serpiginous thick margins of low signal on T1-weighted and high signal on T2-weighted images (viable tissue) or with margins of low signal on both T1 and T2 imaging (necrotic tissue) [10]. The double-line sign, consisting of an outer line of low signal and an inner line of high signal on T2-weighted sequences, is considered virtually diagnostic of osteonecrosis [10]. These MRI features are presumed to represent late stage I osteonecrosis [8]. However, before the development of these features, our cohort showed normal marrow areas demarcated by an indistinct thin single winding line appearing hypointense on T1 sequences and hyperintense on T2 STIR sequences. We postulate that these signal changes are features of pre–stage I osteonecrosis. This hypothesis was supported by the evolution of these subtle signal changes to the more recognized features of osteonecrosis in all patients on follow-up examinations. Furthermore, in 10 of 20 cases (50%), the frank osteonecrotic lesions were confined to the outlines and contours of the early signal changes.
In 10 of 20 knees (50%) and all three hips, the osteonecrosis that evolved was far more extensive than the early signal changes, involving larger areas of the metaphysis, diaphysis, or the epiphysis. In five of seven knees (71%) and all three hips, the epiphysial signal changes increased from 0% to less than 30% or from less than 30% to 30% or more within a mean period of 4.4 months. This finding appears to contradict those of Karimova et al. [14], who reported no change in the size or location of typical osteonecrotic lesions between initial diagnosis and the most recent follow-up examination over a 10-year period. Although we observed no change in the size of frank osteonecrotic lesions on further follow-up (third and fourth follow-up MRI examinations), the frank osteonecrosis that evolved was much more extensive than indicated by the initial signal changes. This observation may reflect either rapid progression of the lesions or poor detectability of the initial signal changes, especially in the diaphysis. Nevertheless, these signal changes were well visualized within the epiphysis in our cohort and could be used to identify patients whose joints require closer monitoring. Core decompression or nonsurgical interventions, such as avoidance of weight bearing, pharmacologic therapy, extracorporeal shock wave therapy, electromagnetic stimulation, and physical therapy, could be evaluated in patients with these early lesions to determine whether intervention at this stage would preserve joint integrity [4–6].
Male sex, age older than 10 years, skeletal maturity, and osteonecrotic involvement of more than 30% of the epiphysial articular surface is associated with articular collapse [13–16]. In our cohort, signal changes were seen to involve epiphyses in 17 (85%) and metaphyses in 18 of 20 (90%) patients. Of the 13 knees with 30% or more epiphysial articular signal changes, only two (15%) eventually showed articular collapse. One knee joint with no initial epiphysial signal changes subsequently showed osteonecrotic involvement of 30% or more of the epiphysial articular surface that progressed to collapse, whereas both hip joints that progressed to collapse showed initial signal changes over 30% of the epiphysial articular surface. Although the five patients who eventually developed articular collapse met the reported criteria for the development of joint articular collapse (i.e., four of the five were boys, three were skeletally immature, and four had more than 30% epiphysial articular surface signal changes), lack of progression to articular collapse in the remaining 11 knees meeting similar criteria may have been the result of the limited duration of follow-up. Furthermore, the subtlety of the signal abnormalities may have obscured their localization along the articular surface. In contrast to the 10-year follow-up reported by Karimova and colleagues [14], most of our cases were followed for approximately 2 years, which may have reduced our detection of progression. Involvement of the articular surface was more easily discerned after development of frank osteonecrosis, and the probability of joint collapse could then be determined on the basis of established criteria. Notably, 15 of our 21 patients were skeletally immature, although age younger than 10 years and skeletal immaturity have been reported to reduce the likelihood of osteonecrosis [16, 17].
Our study was limited by the small number of patients who had these early MRI signal changes. Although of a limited duration of follow-up, this study shows the rapid progression of the described MRI signal changes. Because joint function was not clinically assessed, collapse of the articular surface was our only indicator of clinical outcome; the prognosis of joint integrity and the clinical impact of these signal changes could not be evaluated.
In conclusion, we describe MRI findings that predict the development of osteonecrosis earlier and of greater extent of involvement than indicated by currently recognized MRI changes. Longer-term studies of larger patient cohorts will be necessary to confirm our findings and determine the role of MRI in developing specific clinical interventions.
Acknowledgments
We thank Kimberly Johnson for data management, Sharon Naron for editing of the manuscript, and Sandra Gaither for manuscript preparation.
This work was supported in part by the National Institutes of Health (grant P30 CA-21765), a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities.
References
- 1.Pui CH, Pei D, Campana D, et al. Improved prognosis for older adolescents with acute lymphoblastic leukemia. J Clin Oncol. 2011;29:386–391. doi: 10.1200/JCO.2010.32.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojala AE, Lanning FP, Pääkkö E, Lanning BM. Osteonecrosis in children treated for acute lymphoblastic leukemia. Med Pediatr Oncol. 1997;29:260–265. doi: 10.1002/(sici)1096-911x(199710)29:4<260::aid-mpo5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Kaste SC, Shidler TJ, Tong X, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 4.Marker DR, Seyler TM, McGrath MS, Delanois RE, Ulrich SD, Mont MA. Treatment of early stage osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90(suppl 4):175–187. doi: 10.2106/JBJS.H.00671. [DOI] [PubMed] [Google Scholar]
- 5.Mont MA, Baumgarten KM, Rifai A, Bluemke DA, Jones LC, Hungerford DS. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82:1279–1290. doi: 10.2106/00004623-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Nam KW, Kim YL, Yoo JJ, Koo K-H, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90:477–484. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg ME, Brighton CT, Corces A, et al. Osteonecrosis of the femoral head: results of core decompression and grafting with and without electrical stimulation. Clin Orthop Relat Res. 1989;249:199–208. [PubMed] [Google Scholar]
- 8.Markisz JA, Knowles RJ, Altchek DW, Schneider R, Whalen JP, Cahill PT. Segmental patterns of avascular necrosis of the femoral heads: early detection with MR imaging. Radiology. 1987;162:717–720. doi: 10.1148/radiology.162.3.3809485. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell MD, Kundel HL, Steinberg ME, Kressel HY, Alavi A, Axel L. Avascular necrosis of the hip: comparison of MR, CT, and scintigraphy. AJR. 1986;147:67–71. doi: 10.2214/ajr.147.1.67. [DOI] [PubMed] [Google Scholar]
- 10.Saini A, Saifuddin A. MRI of osteonecrosis. Clin Radiol. 2004;59:1079–1093. doi: 10.1016/j.crad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Gillespy T, Genant HK, Helms CA. Magnetic resonance imaging of osteonecrosis. Radiol Clin North Am. 1986;24:193–208. [PubMed] [Google Scholar]
- 12.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karimova EJ, Rai SN, Ingle D, et al. MRI of knee osteonecrosis in children with leukemia and lymphoma. Part 2. Clinical and imaging patterns. AJR. 2006;186:477–482. doi: 10.2214/AJR.04.1597. [DOI] [PubMed] [Google Scholar]
- 14.Karimova EJ, Wozniak A, Wu J, Neel MD, Kaste SC. How does osteonecrosis about the knee progress in young patients with leukemia? A 2- to 7-year study. Clin Orthop Relat Res. 2010;468:2454–2459. doi: 10.1007/s11999-010-1358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelbrecht V, Scherer A, Bruder M, Körholz D, Mödder U. MRI of aseptic osteonecrosis in children and adolescents with acute lymphoblastic leukemia [in German] Rofo. 2000;172:336–341. doi: 10.1055/s-2000-335. [DOI] [PubMed] [Google Scholar]
- 16.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22:3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Nachman JB, La MK, Hunger SP, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27:5189–5194. doi: 10.1200/JCO.2008.20.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]